Abstract
Intestinal immune cells are important in host defense, yet the determinants for human lymphoid homeostasis in the intestines are poorly understood. In contrast, lymphoid homeostasis has been studied extensively in mice, where the requirement for a functional common γ-chain molecule has been established. We hypothesized that humanized mice could offer insights into human intestinal lymphoid homeostasis if generated in a strain with an intact mouse common γ-chain molecule. To address this hypothesis, we used three mouse strains (non-obese diabetic (NOD)/severe-combined immunodeficient (SCID) (N/S); NOD/SCID γ-chain−/− (NSG); and Rag2−/− γ-chain−/− (DKO)) and two humanization techniques (bone marrow liver thymus (BLT) and human CD34+ cell bone marrow transplant of newborn mice (hu)) to generate four common types of humanized mice: N/S-BLT, NSG-BLT, NSG-hu, and DKO-hu mice. The highest levels of intestinal human T cells throughout the small and large intestines were observed in N/S-BLT mice, which have an intact common γ-chain molecule. Furthermore, the small intestine lamina propria T-cell populations of N/S-BLT mice exhibit a human intestine-specific surface phenotype. Thus, the extensive intestinal immune reconstitution of N/S-BLT mice was both quantitatively and qualitatively better when compared with the other models tested such that N/S-BLT mice are well suited for the analysis of human intestinal lymphocyte trafficking and human-specific diseases affecting the intestines.
Similar content being viewed by others
Introduction
Despite the importance of intestinal immune cells in host defense against luminal pathogens, little is known regarding the factors that contribute to human lymphoid homeostasis in the intestines in health or in disease. Extensive research on the gastrointestinal immune system in mice has led to significant progress in our understanding of the molecular basis of intestinal reconstitution with immune cells. In particular, the interleukin (IL)-2 receptor (common) γ-chain has been found to be essential for the population of the mouse intestines with lymphoid cells.1, 2, 3, 4, 5, 6 Yet, it remains unknown whether human cells depend on the common γ-chain for efficient trafficking of lymphoid cells into the intestines and for the establishment of gut-associated lymphoid tissue. As human and mouse intestines are closely related in both anatomy and physiology,7 we utilized humanized mice to address this question in a model where human T-cell trafficking into the intestines could be examined in vivo. Humanized mice are immunodeficient animals harboring human immune cells generated in situ as a result of a human CD34+ hematopoietic stem cell bone marrow transplant. Humanized mice serve as a useful research platform to probe human intestinal development questions that cannot be addressed directly in humans.
The transplantation of human CD34+ hematopoietic stem cells into severe-combined immunodeficient (SCID) or non-obese diabetic (NOD)/SCID mice resulted in de novo generation of human B cells, monocytes/macrophages, and dendritic cells.8, 9 A major limitation of these humanized mouse models, however, was the total absence of human T cells.8 Additional immunodeficient mouse strains were developed that lacked the mouse common γ-chain that is required for signaling through the mouse IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 receptors.10, 11, 12, 13, 14, 15, 16, 17, 18 As with NOD/SCID (N/S) mice, common γ-chain-deficient mice (NOD/SCID γ-chain−/− (NSG) and Rag2−/− γ-chain−/− (DKO)) are efficiently engrafted by human CD34+ cells that give rise to human B cells, monocytes/macrophages, and dendritic cells. Importantly and in contrast to N/S mice, NSG and DKO mice are capable of supporting human T-cell development.10, 11, 12, 13, 14, 15, 16, 17, 18
The development of humanized mice harboring de novo generated human T cells was a major advance in the field, but it should be noted that in these models human T cells are produced in the context of a mouse thymus with epithelial cells expressing mouse major histocompatibility complex molecules. A third generation of humanized mice was then developed that included the presence of a bona fide human thymus.19, 20 These mice are created by performing a bone marrow transplant of autologous human CD34+ cells into mice implanted with autologous human liver and thymus tissue under the kidney capsule (as with SCID-hu thymus and liver mice).19, 20, 21 In bone marrow liver thymus (BLT) mice, human B cells, monocytes/macrophages, dendritic cells, and thymocyte precursors are produced by the bone marrow, whereas human T cells are generated in the implanted human thymus. In these mice, the human thymocytes produced in the context of a human thymic epithelium become T cells that are capable of mounting human leukocyte antigen-restricted immune responses.20, 22, 23
In mice, the importance of the mouse common γ-chain for intestinal lymphoid tissue development has been definitively shown; mice lacking the common γ-chain do not develop gut-associated lymphoid tissue when compared with wild-type animals.1, 2, 3, 4, 5, 6 Nevertheless, human hematopoietic stem cell transplantation of such strains results in high levels of human cell engraftment, a characteristic highly desirable for the production of humanized mice.24, 25 We hypothesized that an unintended consequence of the humanization of mice lacking the common γ-chain molecule would be inefficient reconstitution of the humanized mouse intestines with human T cells. To address this question, we generated four types of humanized mice extensively used in biomedical research: three that are based on common γ-chain-deficient mice and one that is based on a mouse strain with an intact common γ-chain (Figure 1). We then examined their intestines to compare directly the levels of reconstitution with human T cells between models. Consistent with our hypothesis, the results of this comparison are that the highest immune reconstitution of the mouse intestines with human T cells is observed in mice expressing the common γ-chain molecule. Furthermore, we also observed that the presence of a human thymus further improves the levels of reconstitution of the mouse intestines with human T cells.
Schematic representation of the strains and humanization protocols utilized to generate humanized mice for this comparative analysis. The top row depicts the three immunodeficient mouse strains that were used for this analysis. Non-obese diabetic (NOD)/severe-combined immunodeficient (SCID) (N/S) mice have an intact mouse γ-chain molecule, whereas NOD/SCID γ-chain−/− (NSG) and Rag2−/− γ-chain−/− (DKO) mice lack the mouse common γ-chain. Each strain was preconditioned with the optimized dose of radiation before the transplant of human CD34+ cells as part of either the bone marrow liver thymus (BLT) or transplant-only humanization protocols shown. The identifying label associated with each of the four humanized mice analyzed is indicated.
Results
Humanized mice are generated in several different mouse strains by multiple techniques (Figure 1). NOD/SCID, NSG, and DKO are the three immunodeficient mouse strains most frequently used for the production of humanized mice and each has specific technical requirements for achieving the maximum human engraftment possible. Therefore, we implemented previously optimized and published techniques for generating maximal human engraftment in each of the different humanized mice utilized here to produce the highest likelihood of observing intestinal human T-cell reconstitution in each type of humanized mouse examined.11, 12, 20, 26, 27, 28, 29, 30, 31, 32 Human CD34+ hematopoietic stem cell transplantation into preconditioned recipients is a common aspect of the techniques employed for generating the humanized mice tested here.25 However, to generate BLT mice, the human CD34+ cell transplant is performed in conjunction with surgical implantation of autologous fetal thymus and liver tissue that develops into a bona fide human thymus under the mouse kidney capsule.19, 20 In total, we generated four of the most commonly utilized types of humanized mice: N/S-BLT, NSG-BLT, NSG-hu, and DKO-hu (Figure 1).24, 25
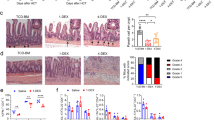
Regardless of mouse strain or humanization protocol, the human CD34+ cells that engraft the bone marrow of humanized mice are known to give rise to human T, B, monocytes/macrophages, natural killer, and dendritic cells that systemically repopulate these animals.24, 25 For the humanization analyses presented in this manuscript ( Tables 1, 2 and 3), we examined 27 humanized mice (N/S-BLT, n=6; NSG-BLT, n=10; NSG-hu, n=7; and DKO-hu, n=4). The average peripheral blood human CD45-expressing cells among these mice was 33% (2–86% range; n=27). Consistent with previous reports,24, 25 we observed human hematopoietic cells, including CD3+ T cells, in bone marrow, spleen, liver, and lungs of N/S-BLT, NSG-BLT, NSG-hu, and DKO-hu mice ( Table 1 and Figure 2a). Mann–Whitney U-test comparisons were performed comparing the absolute numbers of human T cells found in the four types of humanized mice within each of the tissues analyzed ( Table 1). Of these 24 tests conducted, eight comparisons showed significant differences between groups. Seven of the eight tests revealed statistically significant lower levels of humanization in DKO-hu mice relative to the other three types of humanized mice. Between the NSG-hu, NSG-BLT, and N/S-BLT mice, there were fewer differences in human T-cell levels in these tissues. These comparisons presented in Table 1 confirm previously published humanization comparison data with related humanized mice.33 Taken together, these results demonstrate the presence of human reconstitution in primary lymphoid organs and secondary lymphoid organs, liver and lungs in these four types of humanized mice regardless of whether the human T cells were produced in a human or a mouse thymus (Figure 3).
Human engraftment is similar in the lungs and mesenteric lymph nodes (LNs) of non-obese diabetic (NOD)/severe-combined immunodeficient (SCID)-bone marrow liver thymus (N/S-BLT), NOD/SCID γ-chain−/− (NSG)-BLT, NSG-human CD34+ cell bone marrow transplant of newborn mice (hu), and Rag2−/− γ-chain−/− (DKO)-hu mice, but intestinal human engraftment differs between these groups. (a–d) Immunohistochemistry was used to identify human cells in the lungs, mesenteric lymph nodes, and intestines of humanized mice. (a, b) Human CD45+ hematopoietic cells are present in the lung (a) and the mesenteric LNs (b) of each type of humanized mouse. (c, d) Human CD45+ cells were readily detected in the intestines of N/S-BLT, NSG-BLT, and NSG-hu mice, but not in the intestines of DKO-hu mice (Bar=100 μm). Lg, large; Sm, small.
The humanization strategy and mouse strain determine whether a mouse thymus, a human thymus, or both are present, wherein human T cells are produced. In non-obese diabetic (NOD)/severe-combined immunodeficient (SCID)-bone marrow liver thymus (N/S-BLT) mice, all human T cells are produced in the human thymic organoid. NOD/SCID γ-chain−/− (NSG-BLT) mice have both a human thymic organoid and a mouse thymus. NSG-human CD34+ cell bone marrow transplant of newborn mice (hu) and Rag2−/− γ-chain−/− (DKO)-hu mice have only the mouse thymus as they do not contain surgically implanted human tissues. The ages of the human grafts at the time of analysis were between 13 and 23 weeks post-transplant. Table 2 provides absolute numbers of human thymocytes present in each type of humanized mouse shown in this figure.
The mouse strain, combined with humanization protocol, determines whether there is the potential for human T-cell production to occur only in a human thymus, only in a mouse thymus, or in both the human thymus and mouse thymus within the same mouse (Figure 3). Both the human thymus and the mouse thymus in humanized mice generate diverse T-cell receptor (TCR) Vβ repertoires.18, 20, 34 Thus, the impacts of the specific thymus or thymuses functioning in a particular type of humanized mouse are based on the total number of thymocytes present ( Table 2) and also on T-cell education. Specifically, the thymic stroma differs between human and mouse thymuses, resulting in either human leukocyte antigen-restricted and/or mouse major histocompatibility complex-restricted human T cells depending on the specific humanized mice analyzed.16, 20, 22, 23 While only the mouse thymus is present in NSG-hu and DKO-hu mice,10, 16, 17, 18 N/S-BLT and NSG-BLT mice have an implanted human thymus where human T cells are produced (Figure 3).20, 22, 23 N/S-BLT mice have a vestigial mouse thymus devoid of lymphocytes; thus, all human T cells are produced by the implanted human thymic organoid.20 The NSG-BLT mice present an interesting situation because human T cells can be produced in a human or a mouse thymus in parallel in the same mouse (Figure 3).
Given the critical contribution of mesenteric lymph nodes to immune cell migration into the intestines,35, 36 we examined the ability of the human cells to populate the mesenteric lymph nodes in each of these types of humanized mice. Human cells were found throughout the mesenteric lymph nodes of mice from all four models (Figure 2b). We then proceeded to determine whether human cells are present in the intestines in each of the four different types of humanized mice. Human cells were readily detected in the small (SI) and large intestines (LI) of N/S-BLT mice (Figure 2c and d). Similarly, the SI and LI of NSG-BLT and NSG-hu mice were found to harbor human CD45+ hematopoietic cells (Figure 2c and d). In contrast, human cells in the intestines of DKO-hu mice were rarely observed (Figure 2c and d).
Effector sites of the SI and LI include the lamina propria and the single layer of epithelium adjacent to the lumen supported by the lamina propria. Lymphocytes found within these sites are referred to as either lamina propria lymphocytes (LPL) or intraepithelial lymphocytes (IEL) based on their location. We determined the numbers of human T cells in the entire gut and in each of these intestinal compartments: SI LPL, SI IEL, LI LPL, and LI IEL. There were clear differences in the intestinal human T-cell levels noted between the different types of humanized mice analyzed with the N/S-BLT mice exhibiting the highest levels of intestinal human T cells of all humanized mouse groups ( Table 3). Taken together, the immunohistochemistry and flow cytometry analyses confirm differences in human T-cell engraftment of the intestines of different types of humanized mice (Figure 2c and d and Table 3).
The data obtained from the intestinal analyses demonstrate that N/S-BLT mice exhibit high and consistent levels of human T cells throughout all the intestinal fractions ( Table 3). N/S mice have wild-type mouse common γ-chain, but NSG mice are deficient for this gene (Figure 1). Both N/S-BLT and NSG-BLT mice have a human thymus (Figure 3 and Table 2). Our data show that the intestines of N/S-BLT and NSG-BLT mice harbor similar levels of human T cells in the SI LPL, but not the LI LPL ( Table 3). In addition, the levels of T cells in the N/S-BLT SI IEL and LI IEL are significantly higher than those observed in NSG-BLT intestines ( Table 3), which may reflect increased proliferation or longer survival of human T cells in the IEL space of N/S-BLT mice. Regardless of the mechanism, these significant differences demonstrate the critical requirement for the mouse common γ-chain in obtaining robust levels of human intestinal T cells in humanized mice, as observed in N/S-BLT mice.
As indicated above, NSG-hu mice only have a mouse thymus, whereas NSG-BLT mice have a human and a mouse thymus (Figure 3). The most notable effect of this difference is the significantly higher level of human T cells in the SI LPL fraction found in NSG-BLT mice ( Table 3). Comparisons of the absolute numbers of intestinal human T cells found in NSG-BLT to NSG-hu mice revealed that the human T-cell levels were different in the SI LPL and LI IEL, but not in the SI IEL or LI LPL ( Table 3). Furthermore, DKO-hu mice had the lowest levels of human T cells in the SI LPL, SI IEL, LI LPL, and LI IEL when compared with N/S-BLT, NSG-BLT, and NSG-hu mice. Taken together, these data indicate that both the presence of a human thymus and the presence the γ-chain molecule contribute to higher levels of human T cells in the intestines of humanized mice.
In addition to the contributions from the mouse common γ-chain and the human thymus, another contributing factor in the reconstitution of the mouse intestines with human T cells could be differences in the expression of homing molecules that direct T cells to the intestines. In mice, α4β7 integrin has been shown to play an important role in T-cell trafficking to the intestines.7, 37, 38 In humans, peripheral blood T cells frequently express α4 and β7 on their surface and these two integrin subunits form a heterodimer in the presence of retinoic acid imprinting them for trafficking into the intestines.39 β7 Expression on the surface of peripheral lymphocytes is an established marker to demonstrate the potential for intestinal homing of human T cells.40 We therefore determined the levels of human CD8+β7+ and CD4+β7+ T cells in each type of humanized mice (Figure 4). We examined β7 expression because antibodies specific for the human α4β7 heterodimer are not available and co-expression of α4 and β7 on the surface of any given cell does not indicate whether the α4β7 heterodimer is actually present. We observed comparable levels of β7+ human T cells in all four types of humanized mice (Figure 4). The similar levels of β7+ human T cells in all types of humanized mice indicates that differences in homing receptor expression among humanized mice is likely not responsible for the different levels of human T cells present in the intestines.
Non-obese diabetic (NOD)/severe-combined immunodeficient (SCID)-bone marrow liver thymus (N/S-BLT), NOD/SCID γ-chain−/− (NSG)-BLT, NSG-human CD34+ cell bone marrow transplant of newborn mice (hu), and Rag2−/− γ-chain−/− (DKO)-hu mice have comparable levels of β7+ blood T cells. All humanized mice examined harbor human CD4+β7+ and CD8+β7+ T cells in their peripheral blood indicating their potential to traffic to the intestines. In all, 12 Mann–Whitney U-tests were performed comparing results from the four types of humanized mice, of which 10 tests showed no significant difference (α=0.05; unmarked). The two comparisons yielding significant differences are represented by a line connecting the arrows above the respective bars (*P<0.05; **P<0.01).
We sought to determine the extent that human T cells in humanized mouse intestines are similar to intestinal T cells in normal human gut. Since the highest human T-cell reconstitution levels occurred in N/S-BLT and NSG-BLT mice, we focused these detailed analyses in the SI of these two types of humanized mice. We previously reported that the human intestinal T cells in N/S-BLT mice29 and NSG-BLT mice28 exhibit a memory surface phenotype just as in human intestines, leading us to explore additional phenotypes to be compared between these BLT mice and human intestines. Intestinal flora is present in normal humans, but their intestinal T cells do not produce effector molecules (e.g., perforin, granzyme B).41 Similarly, we observed no perforin or granzyme B production in the SIs of N/S-BLT and NSG-BLT mice using a similar immunohistochemistry approach to that reported for the human studies. Thus, the intestinal T cells in these humanized mice are acting in a manner analogous to a normal human gut, suggesting that they are tolerized to the intestinal flora recapitulating a critical human intestinal T-cell phenotype. Next, we examined the TCR Vβ repertoire in the SIs of these humanized mice. Given that their intestinal mucosa and sub-mucosa is comprised of a mixture of mouse epithelial cells, mouse stromal cells, and human leukocytes, this environment could result in the skewing of T-cell populations. This appeared not to be the case as our results show that the human TCR Vβ repertoire in the SI IEL and SI LPL fractions of both N/S BLT and NSG-BLT mice were polyclonal, as has been described in young humans (Figure 5).42 For a third comparison between normal human intestinal T cells and those in humanized mice, we took advantage of the fact that human intestinal T cells have an unambiguous surface phenotype that distinguishes them from human T cells in other tissues in the body. Specifically, on the surface of the CD8+CD4− T cells in the human SI, the CD8 receptor is typically a heterodimer composed of a CD8α molecule plus a CD8β molecule; however, on the surface of human intestinal CD8+CD4+ T cells, the CD8 receptor is typically composed of a homodimer of two CD8α chains (CD8αα).43, 44 We found that the expression of CD8αα on CD8+CD4+ T cells described in human intestines is also observed on the SI LPL and SI IEL cells in N/S-BLT mice (Figure 6a, c, and d). In contrast, cells expressing this homodimer were essentially absent from NSG-BLT mice (Figure 6b–d). Given that both N/S-BLT and NSG-BLT mice have a human thymus, this observation indicates that the mouse common γ-chain molecule contributes to the reconstitution of the N/S-BLT mouse intestines with the same human T-cell populations that are present in human intestines.
T-cell receptor (TCR) Vβ repertoires are diverse in the small intestines (SIs) of non-obese diabetic (NOD)/severe-combined immunodeficient (SCID)-bone marrow liver thymus (N/S-BLT) and NOD/SCID γ-chain−/− (NSG)-BLT mice. (a–d) Immunoscope analysis of pooled SI intraepithelial lymphocytes (IEL) and SI lamina propria lymphocytes (LPL) fractions of (a, c) N/S-BLT (n=4) (b, d) NSG-BLT (n=7) revealed a polyclonal TCR Vβ repertoire in each fraction analyzed. Notably, each Vβ from 2 to 30 was identified in each intestinal fraction examined. Pie charts detail the relative proportions of each Vβ to the total number of amplicons within each fraction.
CD8αα+CD4+ human T cells are present in the small intestine (SI) of bone marrow liver thymus (N/S-BLT) mice, but not NOD/SCID γ-chain−/− (NSG)-BLT mice. (a) As in humans, N/S-BLT mouse CD8+CD4+ human T cells from the SI lamina propria primarily express an intestine-specific CD8αα homodimer, while the CD8+CD4− human T cells typically express the CD8αβ heterodimer. (b) Unlike in humans, NSG-BLT mouse SI lamina propria human T cells primarily express CD8αβ heterodimers on both CD8+CD4+ and CD8+CD4− cells. (c) CD8αα homodimer expression was similar on CD8+CD4− T cells of the SI lamina propria lymphocytes (LPL) and SI intraepithelial lymphocytes (IEL) fractions from N/S-BLT and NSG-BLT mice. (d) In contrast, t-tests indicated that CD8αα homodimer expression was significantly different on CD8+CD4+ T cells of the SI LPL and SI IEL fractions from N/S-BLT and NSG-BLT mice (^SI IEL fractions from three mice were analyzed, but only two of the three had detectable human T cells and were included in these analyses.) These differences are represented by lines connecting the arrows above the respective bars (*P<0.05; **P<0.01) reflecting the qualitative differences between these models demonstrated by the SI T cells in N/S-BLT mice exhibiting an intestine-specific human surface phenotype.
Discussion
Intestinal immune cells are a critical component of a normal human immune system and the availability of a small animal model where the intestines are populated with human T cells will aid in the study of human intestinal immune development, intestinal lymphocyte trafficking, and the pathogenesis of human diseases affecting the intestines.7 To determine if humanized mice could help fill this need, we conducted a comparative study of human T-cell reconstitution in the intestines of four of the most commonly utilized types of humanized mice: N/S-BLT, NSG-BLT, NSG-hu, and DKO-hu mice.24, 25
DKO-hu mice were the first humanized mouse model where the transplant of human CD34+ cells was shown to result in systemic population with de novo generated human T cells.15, 18 This frequently utilized humanized mouse model24, 25 has been shown to harbor human intestinal immune cells,12, 32 although this finding has been contradicted.14 We directly addressed this conflicting literature by examining the intestinal human reconstitution in DKO-hu mice. To maximize the potential for identifying human reconstitution in DKO-hu mice, we cultured the CD34+ cells overnight in cytokines before transplantation into DKO mouse pups according to the protocols reporting the highest levels of intestinal immune reconstitution in this model.11, 12, 32 We identified human T cells in DKO-hu mouse bone marrow, spleen, liver, mesenteric LN, and lungs, a mucosal tissue distinct from the intestines ( Table 1 and Figure 2a and b). However, it is important to note that very few human intestinal T cells were identified in DKO-hu mice (Figure 2c and d and Table 3). When compared with NSG-hu mice, the intestinal human T-cell reconstitution of DKO-hu mice was lower (Figure 2c and d and Table 3). Similarly, human T-cell levels also differed between DKO-hu and NSG-hu mice in the bone marrow, liver, and lungs ( Table 1). These results confirm previously published data showing that immunodeficient mice derived from BALB/c backgrounds humanize less efficiently relative to those derived from NOD backgrounds, likely due to signal regulatory protein α polymorphisms between the founder strains.33, 45, 46, 47 In addition to DKO-hu and NSG-hu mice, we also determined the intestinal human T-cell levels in two distinct BLT humanized mice generated in immunodeficient strains derived from NOD founders, NSG-BLT and N/S-BLT mice.
NSG-BLT and NSG-hu mice are generated in the same immunodeficient mouse strain lacking the mouse common γ-chain molecule (NSG). This critical distinction permits a determination of the effect of BLT humanization on intestinal T-cell levels. We found that these two types of humanized mice have comparable human T-cell levels in their spleen, liver, and lungs ( Table 1). While both NSG-BLT and NSG-hu mice have been reported to have intestinal human T cells,13, 28 our comparison revealed that there are more human T cells in the SI LPL fractions of NSG-BLT vs. NSG-hu mice ( Table 3). Importantly, NSG-BLT SI human T cells did not exhibit a human gut-specific phenotype (i.e., CD8αα+CD4+) (Figure 6b–d). The difference in the levels of SI LPL human T cells between NSG-BLT and NSG-hu is likely due to the greater number of human T cells trafficking throughout their body including the mesenteric LN that contribute to the SI LPL population ( Table 2 and Figure 2b).35, 36 The uniqueness of this pair of humanized mice highlights how in NSG mice the human thymus can contribute to higher levels of intestinal T cells, but not their gut-specific phenotype.
Both N/S-BLT and NSG-BLT mice have a human thymus, but only N/S-BLT mice express the mouse common γ-chain molecule. This critical distinction permitted determination of the effect of the mouse common γ-chain on intestinal T-cell levels. We found that these two types of humanized mice have comparable human T-cell levels in their bone marrow, spleen, liver, lungs, and SI LPL ( Tables 1 and 3). We also found that, as in young humans, the SI IEL and SI LPL TCR Vβ repertoires were polyclonal in both N/S-BLT and NSG-BLT mice (Figure 5).42 However, we observed more human T cells in the SI IEL, LI LPL, and LI IEL fractions of N/S-BLT mice vs. NSG-BLT mice ( Table 3). Furthermore, only N/S-BLT mice contain human intestinal CD8αα+CD4+ T cells (Figure 6).43, 44 These differences between N/S-BLT and NSG-BLT mice cannot be explained by differential expression of the β7 integrin component of the intestinal homing receptor, α4β7 (Figure 4). Thus, the uniqueness of this pair of humanized mice emphasizes the critical nature of the mouse common γ-chain for recruiting human gut-specific T cells into the intestines of humanized mice.
The function of the mouse common γ-chain molecule in intestinal immune development provides a putative mechanistic explanation for our observations. Specifically, the mouse common γ-chain molecule is a required component of the mouse IL-7 receptor, which plays an indispensible role in the earliest stages of intestinal immune development.1, 2, 3, 4, 5, 6 Development of the intestinal immune system begins when mouse intestinal epithelial cells produce IL-7 triggering production of lymphotoxins by lymphoid tissue inducer-like cells.1, 2, 48 Lymphotoxin expression leads to an upregulation of the expression of adhesion molecules and chemokines from stromal lymphoid tissue organizer cells that recruit lymphocytes to this site.1, 2, 48 In the immunodeficient mice lacking lymphocytes used to generate humanized mice, this process can only occur after the engrafted human immune system begins to generate human lymphocytes. Without an intact IL-7 receptor on the lymphoid tissue inducer-like cells, as occurs in IL-2Rγ−/−-deficient mice, there is no gut immune development because there is no response to the initial IL-7 produced by intestinal epithelial cells.1, 2, 3, 4, 5, 6 Thus, the general paucity of human intestinal T cells observed in the humanized mice generated in common γ-chain-deficient mice (NSG and DKO) is attributable primarily to the absence of the common γ-chain molecule. The integral nature of the mouse common γ-chain molecule in development of the intestinal immune system clarifies why the most robust and human-like intestinal humanization is observed in N/S-BLT mice and why there are both quantitative and qualitative differences in the intestinal human T-cell reconstitution of N/S-BLT, NSG-BLT, NSG-hu, and DKO-hu mice. These results predict that BLT humanized mice generated in additional strains containing an intact mouse common γ-chain (e.g., NOD/Rag1−/−) would also exhibit robust intestinal T-cell reconstitution.
In conclusion, we found that BLT mice generated in the NOD/SCID strain that contains an intact mouse common γ-chain exhibited the most robust and consistent intestinal immune cell engraftment. In addition, we report that in NSG-BLT mice there are two different sources of human T cells: the mouse thymus and the implanted human thymic organoid. This observation has potentially significant implications for the use of this model to study T-cell function, as T cells are being simultaneously produced in the context of both human and mouse thymic epithelium. The extensive intestinal humanization of N/S-BLT mice and the single source of human T cells produced in the context of a human thymic epithelium make this model an excellent system in which to gain understanding of intestinal lymphocyte trafficking as well as pathogenesis of human-specific diseases affecting the intestines.
Methods
Generation of humanized mice. Our experimental aim was to identify the best humanized mouse model available for the study of intestinal human T-cell levels. Therefore, humanized mice were prepared using published protocols for generating the maximal human engraftment in each type of humanized mice utilized here11, 12, 20, 26, 27, 28, 29, 30, 31, 32 (Figure 1). Human fetal liver (Advanced Bioscience Resources, Alameda, CA)-derived CD34+ cells were isolated using magnetic positive selection. CD34+ cell preparations were analyzed for the presence of human T cells before transplantation (CD3+ cells=0.5% mean; 0–1.3% range) (Supplementary Figure S1 online). BLT mice were generated as described previously.20, 26, 28, 29 Briefly, CD34+ cells were transplanted into preconditioned autologous liver and thymus tissue implanted N/S or NSG mice (NOD.CB17-Prkdcscid/J and NOD.Cg-PrkdcscidIl-2rgtm1Wjl/SzJ, respectively; both from The Jackson Laboratories, Bar Harbor, ME). N/S mice were preconditioned with 325 cGy γ-radiation and transplanted with up to 3 × 106 CD34+ cells. Similarly, NSG mice were preconditioned with 300 cGy γ-radiation and also transplanted with up to 3 × 106 CD34+ cells. This process resulted in the generation of either N/S-BLT or NSG-BLT mice, depending on the specific mouse strain utilized. CD34+ cell transplant-only humanized NSG mice (NSG-hu) were generated as described previously.30, 31 Briefly, up to 3 × 105 purified fetal liver CD34+ cells were injected intrahepatically into 100 cGy preconditioned newborn NSG pups. DKO mice (BALB/c-Rag2−/−γc−/−; DKO) were transplanted with CD34+ cells to generate humanized-DKO (DKO-hu) mice as described previously.11, 12, 32 Briefly, purified fetal liver CD34+ cells were cultured overnight in RPMI 1640 medium containing IL-3, IL-6, and stem cell factor (1, 1, and 2 μg ml−1, respectively) before intrahepatic injection into 400 cGy preconditioned newborn to 3-day-old DKO pups (up to 5 × 105 cells). All mice were maintained either at the Animal Resources Center of the University of Texas Southwestern Medical Center at Dallas or at the Division of Laboratory Animal Medicine at the University of North Carolina at Chapel Hill in accordance with protocols approved by each institution's Institutional Animal Care and Use Committee.
Sample preparation and flow cytometric characterization of human reconstitution. Whole peripheral blood from humanized mice was monitored for human reconstitution according to the BD Biosciences Lyse/Wash protocol (Cat. No. 349202) as we have described previously.20, 28, 29 Following antibody labeling of whole blood, red blood cells were lysed. The remaining cells were washed, fixed, and the sample was analyzed by flow cytometry. Tissue mononuclear cell isolations were also performed according to published methods.20, 28, 29 The absolute numbers of human cells from each type of humanized mouse were calculated by multiplying the total number of cells collected from the indicated tissues by the respective flow cytometry determined cell population percentages. For all flow cytometry analyses, sequential gating of each sample was utilized as described previously.20, 28, 29 In brief, forward and side scatter properties of the samples were used to gate live cells. Within this gate, all cells expressing the human pan-leukocyte marker CD45+ were gated and subsequent gates were then used to identify human CD3+ T cells. Subsets of CD3+ cells were identified based on CD4 and/or CD8 expression. In peripheral blood, human T-cell subsets were examined for human β7 expression as described previously.40 In the SI lamina propria, human T-cell subsets were examined for CD8α and CD8β molecule expression as we described previously.29 This analysis was performed via gating through human CD3ɛ+ cells (α-CD3ɛ clone: SK1) that would permit the detection of both α–β T cells and γ–δ T cells that may be present; however, it should be noted that previous analyses were unable to detect γ–δ cells.29 Cytometry data were collected using a BD FACSCanto cytometer and analyzed using the BD FACSDiva software (v.5.0.2, San Jose, CA).
TCR Vβ repertoire analysis. Immunoscope analysis was performed as described previously to determine the TCR diversity in pooled SI IEL and pooled SI LPL harvested from N/S-BLT (n=4) and NSG-BLT (n=7) mice.49, 50 Briefly, cDNA was prepared from each of the four samples and real-time polymerase chain reaction performed by combining primers for the different Vβ chains (Vβ2–30). Fluorescent products were separated on ABI-Prism 3730 DNA analyzer to determine CDR3 lengths.49, 50
Sample preparation and immunohistochemical characterization of human reconstitution. The harvested tissues were fixed with 4% paraformaldehyde (Sigma, St Louis, MO) overnight and then embedded in paraffin (Leica, Newcastle, UK). Tissue sections (5 μm) were mounted on poly-L-lysine-coated glass slides (Electron Microscopy Sciences, Hatfield, PA) and then dewaxed. Antigen retrieval was performed by heating sections in Diva Decloacker (Biocare Medical, Concord, CA) at 95°C for 30 min. The sections were incubated with mouse anti-human CD45 (1 μg ml−1, clone 2B11+PD7/26; Dako, Glostrup, Denmark) or control mouse IgG1 (1 μg ml−1, clone X 0931; Dako) overnight at 4°C after blocking with Background Sniper (Biocare Medical) for 30 min. Endogenous peroxidase was then blocked with 3% (v v−1) H2O2. After washing, the sections were treated with MACH 3 Mouse Probe (Biocare Medical) for 10 min, followed by MACH 3 Mouse HRP-Polymer (Biocare Medical) for 10 min. The sections were then treated with ImmPACT DAB (Vector, Burlingame, CA) to develop the signals and finally counterstained with hematoxylin (Sigma).
Statistics. All statistical analyses were performed in Prism v.4 (Graph Pad, La Jolla, CA). Mann–Whitney U-tests were utilized to determine differences between groups in Tables 1, 2 and 3 and Figures 4 and 6.
References
Ivanov, I.I., Diehl, G.E. & Littman, D.R. Lymphoid tissue inducer cells in intestinal immunity. Curr. Top. Microbiol. Immunol. 308, 59–82 (2006).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
van de Pavert, S.A. & Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2, 223–238 (1995).
DiSanto, J.P., Guy-Grand, D., Fisher, A. & Tarakhovsky, A. Critical role for the common cytokine receptor gamma chain in intrathymic and peripheral T cell selection. J. Exp. Med. 183, 1111–1118 (1996).
Porter, B.O. & Malek, T.R. IL-2Rbeta/IL-7Ralpha doubly deficient mice recapitulate the thymic and intraepithelial lymphocyte (IEL) developmental defects of gammac−/− mice: roles for both IL-2 and IL-15 in CD8alphaalpha IEL development. J. Immunol. 163, 5906–5912 (1999).
Gibbons, D.L. & Spencer, J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 4, 148–157 (2011).
Cravens, P.D., Melkus, M.W., Padgett-Thomas, A., Islas-Ohlmayer, M., del P Martin, M. & Garcia, J. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells 23, 264–278 (2005).
Hogan, C. et al. Engraftment and development of human CD34(+ )-enriched cells from umbilical cord blood in NOD/LtSz-scid/scid mice. Blood 90, 85–96 (1997).
Hiramatsu, H. et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood 102, 873–880 (2003).
Berges, B.K., Wheat, W.H., Palmer, B.E., Connick, E. & Akkina, R. HIV-1 infection and CD4T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology 3, 76 (2006).
Choudhary, S.K. et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−{gamma}c−/− mouse. J. Virol. 83, 8254–8258 (2009).
Holt, N. et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 28, 839–847 (2010).
Hofer, U. et al. RAG2−/− gamma(c)−/− mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J. Virol. 82, 12145–12153 (2008).
Gimeno, R. et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood 104, 3886–3893 (2004).
Manz, M.G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26, 537–541 (2007).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106, 1565–1573 (2005).
Traggiai, E. et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304, 104–107 (2004).
Lan, P., Tonomura, N., Shimizu, A., Wang, S. & Yang, Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492 (2006).
Melkus, M.W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006).
McCune, J.M., Namikawa, R., Kaneshima, H., Shultz, L.D., Lieberman, M. & Weissman, I.L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241, 1632–1639 (1988).
Brainard, D.M. et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83, 7305–7321 (2009).
Rajesh, D. et al. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rgammanull mice. Hum. Immunol. 71, 551–559 (2010).
Shultz, L.D., Ishikawa, F. & Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Denton, P.W. & Garcia, J.V. Novel humanized murine models for HIV research. Curr. HIV/AIDS Rep. 6, 13–19 (2009).
Denton, P.W. et al. One percent Tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J. Virol. 85, 7582–7593 (2011).
Denton, P.W. et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 5, e8829 (2010).
Denton, P.W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008).
Sun, Z. et al. Intrarectal transmission, systemic infection and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Dash, P.K. et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci. 31, 3148–3157 (2011).
Lepus, C.M. et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 70, 790–802 (2009).
Berges, B.K., Akkina, S.R., Folkvord, J.M., Connick, E. & Akkina, R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/− gammac−/− (RAG-hu) mice. Virology 373, 342–351 (2008).
Brehm, M.A. et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin. Immunol. 135, 84–98 (2010).
Marodon, G. et al. High diversity of the immune repertoire in humanized NOD.SCID.gamma c−/− mice. Eur. J. Immunol. 39, 2136–2145 (2009).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Svensson, M. et al. CCL25 mediates the localization of recently activated CD8alphabeta(+ ) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121 (2002).
von Andrian, U.H. & Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Wagner, N. et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Iwata, M., Hirakiyama, A., Eshima, Y., Kagechika, H., Kato, C. & Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Arthos, J. et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9, 301–309 (2008).
Chott, A. et al. Intraepithelial lymphocytes in normal human intestine do not express proteins associated with cytolytic function. Am. J. Pathol. 151, 435–442 (1997).
Probert, C.S., Saubermann, L.J., Balk, S. & Blumberg, R.S. Repertoire of the alpha beta T-cell receptor in the intestine. Immunol. Rev. 215, 215–225 (2007).
Abuzakouk, M., Carton, J., Feighery, C., O'Donoghue, D.P., Weir, D.G. & O'Farrelly, C. CD4+ CD8+ and CD8alpha+ beta− T lymphocytes in human small intestinal lamina propria. Eur. J. Gastroenterol. Hepatol. 10, 325–329 (1998).
Carton, J., Byrne, B., Madrigal-Estebas, L., O'Donoghue, D.P. & O'Farrelly, C. CD4+ CD8+ human small intestinal T cells are decreased in coeliac patients, with CD8 expression downregulated on intra-epithelial T cells in the active disease. Eur. J. Gastroenterol. Hepatol. 16, 961–968 (2004).
Legrand, N. et al. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc. Natl. Acad. Sci. USA 108, 13224–13229 (2011).
Takenaka, K. et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 8, 1313–1323 (2007).
Strowig, T. et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA 108, 13218–13223 (2011).
Eberl, G. & Littman, D.R. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science 305, 248–251 (2004).
Huntington, N.D. et al. Autonomous and extrinsic regulation of thymopoiesis in human immune system (HIS) mice. Eur. J. Immunol. 41, 2883–2893 (2011).
Lim, A., Lemercier, B., Wertz, X., Pottier, S.L., Huetz, F. & Kourilsky, P. Many human peripheral VH5-expressing IgM+ B cells display a unique heavy-chain rearrangement. Int. Immunol. 20, 105–116 (2008).
Acknowledgements
This work was supported in part by: National Institutes of Health grants AI073146, AI071940, AI082608, AI082637 (J.V.G.), AI081613 (S.K.C.), 5T32AI005284 (J.F.K.), the UNC Center for AIDS Research Grant P30 AI50410, and a Research Fellowship of the Japan Society for the Promotion of Science (T.N.). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. We thank Dr H. Staats for his critical comments regarding this manuscript; Dr J. Estes for his advice regarding the IHC analysis; Ms B Lemercier for her technical assistance with the TCR analysis; Drs M. Chua and N. Kramarcy of the UNC-Chapel Hill Michael Hooker Microscope Facility in for their technical support; Dr A. Rogers and Ms J. Weaver of animal histopathology facility at UNC-Chapel Hill for tissue embedding; the UNC-Chapel Hill Department of Microbiology and Immunology Flow Cytometry Core Facility; the UNC CFAR Biostatistics Core for statistical input; and former and current lab members for their assistance with aspects of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
Supplementary Information is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Denton, P., Nochi, T., Lim, A. et al. IL-2 receptor γ-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol 5, 555–566 (2012). https://doi.org/10.1038/mi.2012.31
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.31
This article is cited by
-
Acute antagonism in three-drug combinations for vaginal HIV prevention in humanized mice
Scientific Reports (2023)
-
A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection
Nature Biotechnology (2023)
-
Highly synergistic drug combination prevents vaginal HIV infection in humanized mice
Scientific Reports (2020)
-
Humanized mouse models of immunological diseases and precision medicine
Mammalian Genome (2019)
-
A human immune system mouse model with robust lymph node development
Nature Methods (2018)