Abstract
Respiratory exposure to antigen induces T cell tolerance via several overlapping mechanisms that limit the immune response. While the mechanisms involved in the development of Treg cells have received much attention, those that result in T cell deletion are largely unknown. Herein, we show that F4/80+ lymph node medullary macrophages expressing TIM-4, a phosphatidylserine receptor, remove antigen-specific T cells during respiratory tolerance, thereby reducing secondary T cell responses. Blockade of TIM-4 inhibited the phagocytosis of antigen-specific T cells by TIM-4 expressing lymph node medullary macrophages, resulting in an increase in the number of antigen-specific T cells and the abrogation of respiratory tolerance. Moreover, specific depletion of medullary macrophages inhibited the induction of respiratory tolerance, highlighting the key role of TIM-4 and medullary macrophages in tolerance. Therefore, TIM-4-mediated clearance of antigen specific T cells represents an important previously unrecognized mechanism regulating respiratory tolerance.
Similar content being viewed by others
Introduction
TIM-4, a member of the T cell, Immunoglobulin, and Mucin domain (TIM) gene family, is located in a genetic region that has been linked to autoimmune and allergic disorders in both mice and humans.1, 2, 3, 4, 5 However, the specific mechanisms by which TIM-4 functions in the immune system are not fully understood. TIM-4 is a receptor for phosphatidylserine (PtdSer) and mediates the phagocytosis of cells that have externalized PtdSer, such as apoptotic cells.6, 7, 8, 9 TIM-4 is highly expressed by resident peritoneal macrophages, marginal zone macrophages, lymph node medullary macrophages and other tissue-associated macrophages, as well as by conventional dendritic cells.6, 10, 11, 12 In contrast to other receptors for PtdSer, expression of TIM-4 is limited to hematopoietic cells, suggesting an important role for TIM-4 in modulating immune responses. Indeed, deficiency of TIM-4 or impairment of TIM-4 function resulted in autoantibodies to double-stranded DNA and elevated levels of serum Ig.7, 12 Moreover, we recently showed that TIM-4 regulates adaptive immunity by mediating the removal of PtdSer-expressing apoptotic antigen-specific T cells, thereby controlling the number of antigen specific T cells that remain after the contraction phase of immune responses.10 These observations suggest that TIM-4 could play an important role in tolerance.
Respiratory tolerance is a state of immunological non-responsiveness induced by exposure to innocuous antigens inhaled in the respiratory tract. In this process, pulmonary dendritic cells sample antigen delivered in the respiratory tract and migrate to the draining bronchiolar and mediastinal lymph nodes, where they present antigen to CD4+ T cells.13, 14, 15 Following encounter with antigen, these T cells undergo an initial phase of antigen-specific activation and expansion, followed by removal of most of the antigen-specific T cells, with the remaining T cell population refractory to antigen rechallenge.15 Thus, T cells undergo one of three possible fates: deletion, anergy, or differentiation into regulatory T cells. These mechanisms function in concert to induce a state of antigen-specific non-responsiveness upon subsequent challenge. The aims of this study were to determine the pathways involved in T cell elimination, and the role of TIM-4 in this process.
Given the role of TIM-4 in modulating T cell numbers remaining after an immune response, we investigated the role of TIM-4 in the deletion of antigen specific T cells during the induction of respiratory tolerance. We show that F4/80+ lymph node medullary macrophages, which express TIM-4, contribute to respiratory tolerance by phagocytosis of antigen specific T cells. Blockade of TIM-4 using either of two different TIM-4 mAbs prevented the induction of respiratory tolerance. Administration of TIM-4 blocking mAb inhibited the phagocytosis of activated and apoptotic antigen specific T cells by medullary macrophages in the lung-draining lymph nodes and led to an increase in the number of antigen-specific T cells without affecting the activation or proliferation of these cells. Depletion of medullary macrophages also prevented the induction of respiratory tolerance, further demonstrating the importance of these cells in respiratory tolerance. These findings suggest a previously unrecognized mechanism whereby respiratory tolerance depends on TIM-4 expressing medullary macrophages, which limit the number of antigen-specific T cells that remain after the induction of respiratory tolerance.
Results
Blockade of TIM-4 prevents the induction of intranasal tolerance
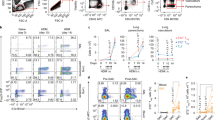
To induce respiratory tolerance, mice were exposed intranasally to 100 μg of LPS-free ovalbumin (OVA) or saline by intranasal instillation on days 0, 1, and 2. The role of TIM-4 in development of tolerance was evaluated by treating with anti-TIM-4 or isotype control antibody on days −1 and 3. Mice were challenged intraperitoneally with OVA in ALUM on day 12. On day 20, T cells from control mice proliferated vigorously after in vitro restimulation with antigen (Figure 1a). In contrast, T cells from mice exposed to intranasal OVA prior to immunization were tolerized and had significantly reduced proliferation and reduced IL-4 and IFNγ responses compared to control mice. However, administration of either of two blocking anti-TIM-4 monoclonal antibodies (QT3.14 or 21H12) reversed the induction of tolerance, resulting in increased proliferative and cytokine responses compared to isotype treated mice (Figure 1a and b). To examine the number of responding cells, splenocytes from anti-TIM-4 treated and control mice were labelled with CFSE prior to culture with OVA. Tolerized mice had a lower percentage of proliferating OVA-specific CD4+ T cells (CFSE low cells) than control mice. However, treatment with anti-TIM-4 mAb prevented tolerance induction, as shown by a restoration of the percentage of CD4+ T cells proliferating to OVA to levels observed in the non-tolerized mice (Figure 1c and d). CFSE labelling also allowed the examination of cytokine production per cell. Cytokine per cell was calculated by dividing total IL-4 or IFNγ production in culture by the number of responding (CFSE low) T cells. No significant differences were found in cytokine per cell between non-tolerized, tolerized, and tolerized plus anti-TIM-4 treated groups (Figure 1e). Thus, in vivo treatment with anti-TIM-4 mAb did not increase levels of cytokine production on a per cell basis. This data suggests that the primary mechanism of tolerance in this model is the deletion of OVA specific cells and that treatment with anti-TIM-4 during tolerance induction impairs this process.
Blockade of TIM-4 inhibits tolerance induction in vivo. (a, b) BALB/c mice injected on days −1 and 3 with anti-TIM-4 mAb (a) QT3.14, or (b) 21H12, (400 μg) or isotype control mAb were exposed on days 0, 1, and 2 to intranasal OVA (tolerized) or saline (nontolerized). All mice were subsequently immunized i.p. with 50 μg OVA adsorbed in 2 mg alum on day 12. On day 20, B depleted splenocytes were restimulated in vitro with OVA and assayed for proliferation and cytokine secretion. Mean and s.e.m. is shown. Statistics compare regression curves fit to the data by F test comparing anti-TIM-4 and isotype treated groups (Figure 1a, P<0.0001). One representative experiment of three is shown, with three mice per group in each experiment. (c, d) B cell depleted splenocytes from mice treated as in (a) were labeled with CFSE and cultured with OVA. On day 4 cultures were stained for CD3 and CD4, and subgated on CD3+CD4+ cells for analysis of CFSE dilution. One representative experiment of three is shown and each group contained four animals per group. (d) Mean and s.e.m. is shown, **P<0.01, ***P<0.001. Student’s t-test used to compare anti-TIM-4 and isotype control treated groups. (e) B cell depleted splenocytes were labeled with CFSE and cultured with OVA. On day 4, cultures were harvested as in (c) and supernatants were collected and assayed for cytokine production. Cytokine per cell is calculated as cytokine amount divided by the number of CFSE low cells. (f) BALB/c mice treated with anti-TIM-4 mAb 21H12 or isotype control were exposed on days 0, 1, and 2 to intranasal OVA. As a positive control, one group of mice were given LPS (100 ng) i.n. with OVA i.n. Draining lymph nodes were harvested 7 days later and cells cultured with OVA. Mean and s.d. are shown. (g) BALB/c mice were tolerized and challenged as in (a); however, anti-TIM-4 or isotype control mAb was administered on day 11. Mean and s.e.m. is shown, difference not significant. Statistics compare regression curves fit to the data by F test comparing anti-TIM-4 and isotype treated groups.
Anti-TIM-4 treatment did not result in priming of T cells, as shown in Figure 1f. Mice were treated with isotype or anti-TIM-4 mAb and given OVA intranasally without subsequent immunization. One group of mice received intranasal OVA plus LPS as a positive control. Cells harvested from the draining lymph nodes on day 9 were restimulated in vitro with OVA. Cells from mice treated with intranasal OVA did not proliferate regardless of treatment with anti-TIM-4 mAb, while as expected, cells from mice that received OVA plus LPS mounted a vigorous response to OVA in vitro. This experiment showed that TIM-4 mAb treatment did not act as an adjuvant and induce priming of T cells.
To confirm that anti-TIM-4 treatment was inhibiting the induction of tolerance to inhaled OVA rather than enhancing the subsequent immune response to challenge with OVA/ALUM, mice were given intranasal OVA to induce tolerance and treated with anti-TIM-4 or control mAb one day prior to immunization with OVA/ALUM. Administration of anti-TIM-4 to tolerized mice at the time of OVA challenge did not restore the response to OVA. Both anti-TIM-4 and isotype treated animals exhibited reduced proliferation and cytokine responses (Figure 1g) compared with non-tolerized mice.
TIM-4 is expressed in the lung draining lymph nodes
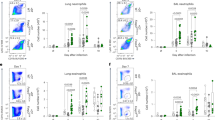
We next examined the cell types that expressed TIM-4 in the lung-draining lymph nodes, where interaction of T cells with APCs occurs. Macrophages in the lung draining lymph nodes, identified as F4/80+CD11b+, expressed TIM-4, and TIM-4 expression levels increased slightly after intranasal treatment with OVA (Figure 2a). Further analysis of these cells showed they express low or no CD11c and do not express Gr1 or B220 (data not shown). Lymph node resident DCs (CD11chi, MHCIIint) also expressed TIM-4, including both CD4 and CD8 DCs (Figure 2b and data not shown). TIM-4 was expressed by both the CD11bhi, CD103low DC subset that presents antigen to CD4+ T cells and the CD11blow, CD103hi DC subset that cross presents antigen to CD8+ T cells.14, 16 (Figure 2b). While the number of DCs in the draining lymph nodes increased after OVA administration, the TIM-4 expression of these DC subsets was unchanged (Figure 2c and data not shown). Lymph node plasmacytoid DCs did not express TIM-4 (Figure 2d), as previously demonstrated for splenic plasmacytoid DCs.6
TIM-4 is expressed by DCs and macrophages in lung draining lymph nodes. BALB/c mice received intranasal saline or OVA on days 0, 1, and 2. Bronchial lymph nodes were harvested on day 3, collagenase digested, and stained for macrophages and dendritic cells. (a) Macrophages were identified as F4/80+CD11b+ and stained for TIM-4 (black) or Isotype (gray). Further analysis of these cells showed they express low or no CD11c and do not express B220 or Gr1. (b) Saline or (c) OVA treated lymph nodes were stained for TIM-4 (black) or Isotype (gray). Single cell suspensions were stained for CD11c, MHCII, B220, CD11b, CD103, and TIM-4/Isotype. Gating on B220− cells allows the clear discrimination of two DC populations CD11chi, MHCIIint and CD11cint, MHCIIhi. CD11cint, MHCIIhi were further subgated using CD11b and CD103 expression. Staining for TIM-4 (black line) compared to isotype control (gray/shaded) is shown. (d) Plasmacytoid dendritic cells were identified as CD11c+, Gr-1+, and stained for TIM-4 (black) or Isotype (gray). (e) BALB/c mice were treated with anti-TIM-4 or isotype control antibody on day −1, and were administered intranasal OVA on days 0, 1, and 2. Bronchial lymph nodes of individual mice were harvested on day 3, and analyzed as in a–d. Cell counts of macrophages and dendritic cells were not significantly different (mean+s.e.m. shown, P>0.05, student’s t-test, n=3). NS=not significant.
We have previously shown that TIM-4 mAbs QT3.14 and 21H12 specifically block the binding and engulfment of apoptotic cells by TIM-4 expressing phagocytic cells.6, 10 To show that anti-TIM-4 21H12 mAb is not a depleting antibody, mice were treated with anti-TIM-4 or isotype control and the number of CD11chi DCs, CD11cint DCs, and F4/80+ macrophages were assessed in the bronchial lymph nodes. Anti-TIM-4 treatment did not alter numbers of macrophages or DCs in the lung draining lymph node (P>0.05 all groups student’s t-test), showing that this antibody does not deplete TIM-4 expressing cells (Figure 2e).
TIM-4+ cells mediate phagocytosis of antigen specific T cells
We used immunofluorescence microscopy to visualize the location of TIM-4+ cells and better understand the processes occurring in the lymph node during tolerance induction. TIM-4+ cells (red) were observed in the medulla and scattered in the T cell zone, with only a few TIM-4+ cells in B cell follicles (Figure 3a and b). During tolerance induction, TIM-4+ cells in the lymph node phagocytosed antigen-specific T cells, as shown by uptake by TIM-4+ cells of adoptively transferred CMFDA (Cell Tracker Green)-labelled OVA specific DO11.10 Rag−/− T cells. One day after the last of three intranasal exposures to OVA, CMFDA labelled DO11.10 T cells (green) were observed within TIM-4+ cells (red) in the draining lymph nodes (Figure 3c and d). Confocal microscopy imaging confirmed the internalization of phagocytosed T cells (green) within a TIM-4+ cell (red) (single confocal XY plane (middle) with an XZ (top) and YZ plane (left)) (Figure 3e). A Z stack movie of this cell shows that the phagocytosed DO11.10 T cell was localized inside the TIM-4+ cell (Supplementary Movie 1 online). A Z stack reconstruction of the surface of this TIM-4+ cell also shows this by comparing 100% surface opacity (Supplementary Figure 1A online) with 50% opacity (Supplementary Figure 1B online), demonstrating remnants of phagocytosed DO11.10 T cells within the TIM-4+ cell. Some TIM-4+ cells were oriented primarily in the Z direction of the microscope, which were best appreciated by a Z stack movie comprised of individual confocal slices (Supplementary Movie 2 online). These results demonstrate that TIM-4+ cells phagocytose DO11.10 T cells in vivo after intranasal OVA administration.
TIM-4 expressing cells phagocytose DO11.10 T cells in the lung draining lymph nodes after intranasal OVA. Sections from bronchial/mediastinal lymph nodes oriented in a manner that allows imaging of B cell zones, T cell zones, and the medulla in a single section. Whole lymph node expression of (a) TIM-4 (red) and (b) B220 (blue) shows reduced TIM-4 expression in the B cell zone and stronger expression of TIM-4 in the T cell zone and in the medulla. Scale bars, 50 μm. (c–g) 106 CMFDA labeled DO11.10 T cells (green) were transferred into naïve hosts which then received 3 intranasal, 100 μg doses of LPS-free OVA. Draining lymph nodes were removed on day 3 and embedded in OCT. Representative confocal images of at least 5 animals are shown. Scale bars are 10 μm. (c–e) Sections were stained with (c, e) anti-TIM-4 (red) or (d) an isotype control antibody (red). (c, d) Maximum Z-projection images are shown. (e) A confocal image of a TIM-4+ cell showing single XY, XZ, and YZ planes showing internalization of DO11.10 T cell material (green) within a TIM-4+ cell. (f, g) TIM-4+ cells that phagocytosed DO11.10 T cells were located near lymphatic endothelium and not in the T cell zone. Lymph node sections were stained for TIM-4 (red) and LYVE-1 (blue). (f) A representative image from the medulla (LYVE-1+ region) is shown with white arrows indicating TIM-4+ cells that have phagocytosed a DO11.10 cell. (g) A representative image from the T cell zone (LYVE-1− region) is shown. DO11.10 T cells in this field are live T cells (not engulfed). (h) Anti-TIM-4 treatment reduced the phagocytosis of activated T cells. Isotype or anti-TIM-4 treated mice received 106 CMFDA labeled DO11.10 T cells after which they received 3 intranasal doses of OVA on days 0, 1, 2. Draining lymph nodes were harvested and stained for TIM-4 and LYVE-1 one day later (day 3). Percentage of medullary TIM-4+ cells that were DO11.10+ was quantified. >100 TIM-4+ cells were counted per mouse. One representative experiment of two is shown. (n=3, **P<0.01 by Student’s t-test).
The majority of the TIM-4+ cells (red) that had phagocytosed DO11.10 T cells (green, white arrows) were located in the lymph node medulla, which is defined by the presence of LYVE-1+ lymphatic endothelium (blue) (Figure 3f). In contrast, few of the TIM-4+ cells in the T cell zone (shown by an absence of LYVE-1 staining) located in the center of the lymph node, phagocytosed DO11.10 T cells (Figure 3g). The DO11.10 T cells in this field are live and have not been engulfed. These findings indicated that TIM-4+ cells residing in the medulla were the primary cells phagocytosing antigen-specific T cells during tolerance induction. Phagocytosis of the DO11.10 T cells was mediated by TIM-4, since treatment of mice with anti-TIM-4 mAb reduced the number of TIM-4+ cells containing DO11.10 T cells in the medulla (Figure 3h).
Blockade of TIM-4 results in increased numbers of antigen specific T cells
Consistent with the idea that there is less phagocytosis of DO11.10 T cells in mice treated with anti-TIM-4 mAb, anti-TIM-4 treated mice had increased numbers of OVA specific T cells remaining in the lymph nodes (Figure 4a). Blockade of TIM-4 had no effect on the early activation and division of the OVA-specific cells, since CFSE dilution of transferred DO11.10 cells was unchanged by anti-TIM-4 treatment (Figure 4b). Uptake of the DO11.10 cells by TIM-4+ macrophages was mediated most likely by PtdSer expression by the DO11.10 cells since a large fraction of the DO11.10 T cells expressed PtdSer (Figure 4c),17 and this was not affected by anti-TIM-4 treatment. These results indicated that TIM-4 mediated the uptake of DO11.10 T cells expressing PtdSer during tolerance induction, but had no effect on the number of cell divisions that the DO11.10 cells underwent.
Anti-TIM-4 treatment increases the number of antigen specific T cells on day 3 after intranasal OVA administration. (a) Isotype or anti-TIM-4 treated mice received 105 CFSE labeled DO11.10 T cells on day 0 and 3 doses of intranasal OVA on days 0,1,2. The number of DO11.10 T cells in draining LN was quantified 3 days after transfer. One representative experiment of three is shown. (n=5, **P<0.01 by Student’s t-test) (b) Although treatment with anti-TIM-4 increased the total number of CFSE labeled DO11.10 T cells in draining lymph nodes (KJ1-26+ cells, black) it did not alter the number of cell divisions. One representative experiment of two is shown. (c) Lymph nodes cells prepared as in (a) were stained for TCRβ, CD4, KJ1-26, and AnnexinV. The black line is gated from CD4+KJ1-26+ cells while the gray, shaded histogram is from endogenous CD4+KJ1-26− cells (same sample) showing that the activated T cells had externalized PtdSer. One representative experiment of two is shown.
Medullary F4/80+, TIM-4+ macrophages phagocytize antigen specific T cells
During respiratory tolerance induction, the DO11.10 T cells were cleared by medullary F4/80+ TIM-4+ macrophages. This was shown by staining lymph node sections from mice that had received CMFDA-labelled DO11.10 T cells with anti-TIM-4, anti-F4/80 and anti-CD11c mAb. TIM-4+ cells that had engulfed DO11.10 T cells costained with anti-F4/80 mAb (Figure 5a) but not with anti-CD11c mAb (Figure 5b), suggesting that the phagocytizing cells were macrophages. These results were confirmed by staining consecutive sections with anti-LYVE-1 and anti-F4/80 mAb. Focusing on a TIM-4+ cell that had phagocytosed green DO11.10 T cells, denoted by the white arrow in Figure 5c we showed that this phagocytic TIM-4+ cell located in the medulla was indeed F4/80+ (Figure 5d). Together, these results show that TIM-4+ macrophages in the lymph node medulla are responsible for the phagocytosis of antigen specific T cells during tolerance induction.
F4/80+ macrophages in the lymph node medulla express TIM-4 and phagocytose DO11.10 T cells. (a–d) 106 CMFDA labeled DO11.10 T cells (green) were transferred into naïve hosts which then received 3 intranasal, 100 μg doses of LPS-free OVA. Lymph node sections were prepared as in Figure 3. Scale bars 10 μm. (a) White arrows indicate colocalization of TIM-4 (red) with F4/80 (blue) (colocalized shows as magenta) on cells that have phagocytosed DO11.10 T cells (green) (triple colocalization shows as white). (b) CD11c (blue) did not colocalize with TIM-4+ cells (red) that had phagocytosed DO11.10 T cells. Colocalization of TIM-4 and DO11.10 appears yellow in cells that do not express CD11c. Representative images of 4 animals shown. (c, d) Consecutive sections were stained for anti-TIM-4 (red) and (c) anti-LYVE-1 (blue) or (d) anti-F4/80 (blue). (c) The white arrow highlights a TIM-4+DO11+ cell located in the medulla (LYVE-1, blue) to be analyzed in (d). (d) A single cell indicated by the white arrow from (c) is shown. Upper left panel is an overlay of all fluorescent channels. Upper right, CMFDA-DO11.10 (green). Lower left, TIM-4 (red). Lower right, F4/80 (blue). Representative images from 2 animals shown. (e) Bronchial lymph nodes were collagenase digested and co-cultured with apoptotic thymocytes labeled with 1.5 μM pHrodo. After two hours, cultures were washed and stained for F4/80, CD11b, and TIM-4. pHrodo is highly fluorescent at low but not neutral pH; therefore, pHrodo+ cells have phagocytosed an apoptotic thymocyte.
To further show that F4/80+TIM-4+ macrophages phagocytose PtdSer-expressing-T cells, bronchial lymph nodes were collagenase digested and single cell suspensions were co-cultured with pHrodo labelled apoptotic thymocytes. The pH sensitive dye pHrodo strongly fluoresces red in acidic conditions, such as the low pH found in a phagosome/endosome but very weakly at neutral pH. Therefore red fluorescence indicates true engulfment as opposed to cell surface binding. Thus, in this assay pHrodo+ cells have phagocytosed an apoptotic T cell. After two hours of co-culture, cells were washed and stained for F4/80, CD11b, and TIM-4. Gating on F4/80+ CD11b+ macrophages shows a TIM-4+ population that phagocytosed apoptotic cells in a dose dependent manner, as shown by pHrodo+ macrophages. This demonstrates that TIM-4 positive macrophages from lymph node can phagocytose apoptotic cells in vitro (Figure 5e).
F4/80+ macrophages are required for respiratory tolerance
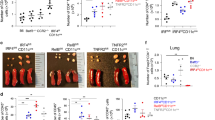
The presence of F4/80+, TIM-4+ medullary macrophages were required for pulmonary tolerance, since treatment of mice with an anti-F4/80 mAb which has been shown to deplete F4/80+ macrophages,18 abolished respiratory tolerance, as shown by examination of proliferation and cytokine responses (Figure 6a). T cells from tolerized mice that received isotype control antibody proliferated poorly in response to OVA restimulation in vitro (Figure 6a) and exhibited reduced IL-4 and IFN-γ cytokine responses compared to the non-tolerized group, indicating the induction of respiratory tolerance (Figure 6a).
Depletion of macrophages by anti-F4/80 abrogates pulmonary tolerance. (a) Tolerance was induced as in Figure 1. Anti-F4/80 or isotype control antibody (250 μg) was administered on days −1, 0, and 1. Mean and s.e.m. is shown. P<0.0001. Statistics compare regression curves fit to the data by F test comparing anti-F4/80 and isotype treated groups. One representative experiment of two is shown. (b) BALB/c mice received anti-F4/80 or isotype control antibody on days −1, 0 and 1, and intranasal OVA on day 0 and 1. Bronchial LN from anti-F4/80 or control treated animals were stained on day 2 for F4/80 (clone BM8 is different clone from depleting antibody), CD11b, CD11c, and MHCII. Gates were established as in Figure 2 and the number of macrophages and DCs was quantified. F4/80+CD11b+ cell were significantly reduced with anti-F4/80 treatment (mean+s.e.m., P<0.01, student’s t-test, n=8). (c) Eosinophils were stained using Siglec-F and F4/80. Total cells were gated on FSClowSSCint characteristic of eosinophils. A population of eosinophils was found in the draining bronchial lymph nodes as Siglec-F+F4/80int. Gate frequency indicates the percentage of FSClowSSCint cells.
Treatment with anti-F4/80 will deplete a number of macrophage subsets, so we determined whether macrophages that express TIM-4 in the medulla of the lung draining lymph nodes were depleted with this treatment. FACS analysis of lymph nodes from control or anti-F4/80 antibody treated animals using a different antibody clone (BM8) to stain for F4/80+ cells showed excellent depletion of F4/80+CD11b+ cells (Figure 6b gated as in Figure 2a). Quantifying numbers of cells in individual mice showed that the number of F4/80+CD11b+ macrophages was significantly reduced (Figure 6b P<0.01, student’s t-test) following treatment with anti-F4/80 compared with control treated mice. Since several other cell types also demonstrate low surface expression levels of F4/80, including dendritic cells, neutrophils, and eosinophils, we also examined numbers of CD11chiMHCIIint DC and CD11cintMHCIIhi DC in the bronchial lymph nodes. Numbers of CD11chi and CD11cint DCs were not significantly reduced after treatment with anti-F4/80 (Figure 6b gated as in, Figure 2b and c P>0.05). Neutrophils were not found in the draining lymph node since Gr1+ cells did not have the forward/side scatter characteristics of neutrophils and are most likely monocytes or pDC (data not shown). Eosinophils were identified by gating on FSCloSSCint cells stained for Siglec-F and F4/80. Eosinophils were found in the draining bronchiole lymph nodes and were not depleted with anti-F4/80 treatment (Figure 6c). DC and eosinophils exhibit low or no cell surface expression of F4/80 and only highly expressing cells appear to be depleted by our treatment regimen. In total, this analysis shows that treatment with anti-F4/80 depletes F4/80+TIM-4+ medullary macrophages and that these cells are required for the induction of pulmonary tolerance. In contrast, DC are not depleted by either anti-F4/80 or anti-TIM-4 treatment in this model.
Histological analysis of the lymph nodes from the treated mice showed that most of the F4/80+TIM-4+ cells were in the medullary area, as noted earlier and as shown by the presence of LYVE-1+ cells (Supplementary Figure 2 online). After treatment with depleting F4/80 mab, F4/80+TIM-4+ cells were mostly absent or displayed a rounded morphology consistent with apoptosis (Supplementary Figure 2 online). The F4/80+ cells were restored several days after anti-F4/80 mAb treatment, presumably as monocyte derived macrophages migrating into the lymph node, but these cells did not express TIM-4. Thus TIM-4, expressed by medullary macrophages, is required for the induction of pulmonary tolerance.
Discussion
In these studies, we demonstrated that TIM-4 expressing lymph node medullary macrophages were required for the induction of respiratory tolerance through a previously unrecognized mechanism involving phagocytosis and clearance of antigen-specific T cells. Thus, treatment with an anti-TIM-4 mAb prevented the induction of respiratory tolerance and blocked the clearance of antigen specific T cells, but had no effect on initial activation and division of the T cells. Phagocytosis of T cells by TIM-4+ cells occurred specifically in the medulla of the lymph nodes, where TIM-4 was expressed by macrophages, and not in the lymph node T cell zone, where TIM-4 is expressed by DCs. Since the depletion of F4/80+ medullary macrophages with an anti-F4/80 mAb also prevented the induction of respiratory tolerance, we conclude that medullary macrophages expressing TIM-4 play a previously unrecognized role in mediating respiratory tolerance.
The general paradigm of respiratory tolerance, which occurs in response to harmless inhaled exogenous antigens, asserts that inhaled antigen is sampled by immature DCs in the lung, which then migrate to the draining mediastinal lymph nodes. In the draining lymph nodes, tolerance is thought to develop through three mechanisms, including anergy, development of regulatory T cells and deletion of antigen-specific T cells, and all three processes may occur concurrently.13, 19, 20, 21 In the T cell zone of the lymph node, DCs first encounter antigen-specific T cells, initiating T cell proliferation and the development of Treg cells, or inducing T cell anergy, due to a lack of costimulation. The T cells then migrate to the lymph node medulla, where our findings suggest that they encounter medullary F4/80+, TIM-4+ macrophages, which bind and remove PtdSer expressing T cells. In contrast, T cells expressing no or much lower levels of PtdSer are allowed to exit the lymph node in the afferent lymphatics. Thus, TIM-4 expressing F4/80+ macrophages play an important role late in the process of tolerance induction, after T cell activation but just before T cells leave the lymph node. It is possible that differential expression of “don’t eat me signals” such as CD47.22 on tolerized vs. activated T cells could also modulate the phagocytosis and deletion of T cells in the lymph node medulla.
We believe that there are various checkpoints along the path to respiratory tolerance and that at these checkpoints, development of anergy, Treg and deletion of antigen-specific T cells may play more or less of a role. Our initial characterization of the respiratory tolerance model showed that deletion was an important mechanism, particularly at 1–2 weeks after tolerance induction.15 In addition, the remaining undeleted OVA-specific T cells were anergic and refractory to antigen challenge,15 indicating that more than one mechanism was involved. Tregs are also important early in the course of respiratory tolerance induction, and we showed that blockade of the costimulatory molecule ICOS prevents Treg development13 and impairs respiratory tolerance. Thus, several mechanisms act in concert to solidify the development of tolerance and prevent immune responses to nonpathogenic inhaled antigens, and interference with one or more of these can impair the development of tolerance.
In the present study we show details of the mechanism responsible for the deletion of antigen specific T cells involved in respiratory tolerance induction. Treatment with anti-TIM-4 prevented development of respiratory tolerance, primarily by blocking the phagocytosis of activated antigen-specific T cells expressing PtdSer by F4/80+ lymph node medullary macrophages. TIM-4-expressing-dendritic cells did not phagocytose activated T cells, although it is possible that a T cell interaction with a TIM-4-expressing DC could affect Treg generation. However, in other studies we found the development of antigen-specific Tregs during an immune response was not affected by anti-TIM-4 treatment,10 suggesting that TIM-4 has little or no effect on Treg development. Although respiratory tolerance involves multiple mechanisms, these findings together with those previously reported, suggest that TIM-4 modulates immune responses primarily by regulating uptake of activated T cells expressing phosphatidylserine.
We postulate that in the lymph node medulla that the TIM-4-expressing macrophages can bind and phagocytize both apoptotic as well as recently activated T cells that have not yet committed to the apoptotic pathway. In other words, the TIM-4 expressing medullary macrophages function not only as a sink for apoptotic cells, but in addition remove PtdSer expressing T cells that might otherwise survive in the periphery. This idea is supported by our observation that blockade of TIM-4 reverses respiratory tolerance by increasing the number of surviving antigen-specific T cells. The binding of both apoptotic and non-apoptotic T cells is mediated by PtdSer, which is externalized by T cells at two stages: during apoptosis with PtdSer levels that are high, and shortly after cell activation,23, 24 with T cells expressing Ptd-Ser levels that are relatively low (Figure 4c). Because TIM-4 has a very high affinity for PtdSer (60 nM),7 which is 25 fold higher than the affinity of MFG-E8 for Ptd-Ser (1600 nM),25 TIM-4-expressing macrophages can phagocytize T cells at both stages, including the recently activated T cells expressing PtdSer over a threshold level. We believe that during tolerance induction the process of binding recently activated T cells prevents the egress of some of the activated antigen-specific T cells from the lymph node. These T cells “arrested” by medullary macrophages may eventually undergo apoptosis due to a lack of survival signals via common γ chain cytokine receptors or through FAS and TRAIL signalling in the lymph node. Thus, F4/80+ macrophages critically influence the degree of T cell clearance by engulfing not only apoptotic-committed T cells, but also early stage PtdSer-expressing T cells not fully committed to apoptosis, thereby contributing to the induction of peripheral tolerance in a previously unrecognized pathway.
The capacity of medullary macrophages to engulf both apoptotic and recently activated T cells supports the idea that the medullary lymphatic sinuses, which are lined by macrophages and DCs, play a critical role in respiratory tolerance. Whereas DCs in the medullary sinuses can take up influenza virus in the lymph and migrate to the T cell zone,26 medullary macrophages do not migrate after exposure to influenza. Rather, it has been observed that medullary macrophages lining the lymph vessel interact with T cells for an extended period of time,27 suggesting that macrophages regulate egress of T cells from the lymph node. Lymphatic sinuses also extend into the cortical region of the lymph node between T cell zones and B cell follicles. These cortical sinuses provide another route of egress from the lymph node,27 In contrast to medullary sinuses, cortical sinuses are not surrounded by macrophages and dendritic cells and we did not observe TIM-4 expressing cells surrounding the cortical sinuses (data not shown). The mechanisms that determine whether a T cell migrates toward a cortical sinus or a medullary sinus have not been investigated. However, the outcome may impact tolerance since macrophages present in the medulla can phagocytose antigen specific T cells, while no such macrophage population lines the cortical sinuses.
Our current examination of TIM-4 in respiratory tolerance extends our studies of how TIM-4 as a receptor for PtdSer regulates immunity. Previously, we showed that TIM-4 expressing macrophages in the spleen mediated the removal of antigen-specific T cells during the resolution phase of influenza infection or antigen immunization.10 During influenza infection, treatment with a blocking anti-TIM-4 mAb had no effect on viral titers but greatly increased the number of influenza-specific memory T cells that remained after the clearance of antigen or infection. The present studies show that in a tolerance model, TIM-4 expressing medullary macrophages regulate the deletion of antigen specific T cells and thus promote non-responsiveness to exogenous antigens. Therefore, TIM-4 functions during both productive immune responses and during peripheral tolerance induction to reduce the number of antigen specific T cells, which highlights the similarities between peripheral tolerance and immunity. Moreover, these studies demonstrate the important role of TIM-4 expressing phagocytic cells in regulating immunity and tolerance, and suggest that further understanding of TIM-4 mediated processes could lead to novel therapies to enhance tolerance induction in the respiratory or intestinal tracts, or to enhance immunity for vaccine or cancer therapy.
Methods
Animals. BALB/cBy mice were purchased from the Jackson Laboratory (Bar Harbor, ME). OVA-specific TCR transgenic DO11.10 Rag2−/− mice were used as donors of OVA-specific CD4+ T cells. The Animal Care and Use Committee, Children’s Hospital Boston approved all animal protocols.
Intranasal tolerance and Mab treatment. To induce tolerance, BALB/cBy mice received 100 μg of LPS free OVA or a saline control by intranasal instillation on days 0, 1, and 2. Mice were administered anti-TIM-4 (clones QT3.14 or 21H126) as indicated or isotype control antibody on days −1 and 3. In some experiments, anti-F4/80 (F4/80 hybridoma obtained from ATCC) or control antibody (250 μg) was administered on days −1, 0, and 1 as described.18 Ten days later mice were immunized with 50 μg OVA adsorbed in 2mg or ALUM. Where indicated, anti-TIM-4 was administered one day prior to immunization with OVA/ALUM instead of prior to tolerance induction. Eight days post immunization, spleens were harvested, depleted of B cells and restimulated in vitro with OVA. Cultures were pulsed with 1 μCi of 3H-thymidine for the final 17 h and harvested at 96 h. Culture supernatants were harvested at 96 h for determination of IFNγ and IL-4 concentrations by ELISA. In some experiments, cells were labelled with CFSE prior to culture and stained on day 4 with CD3 PerCP-Cy5.5, CD4 Alexa 700 and analyzed for CFSE expression.
Transfer of DO11.10 T cells. DO11.10 Rag−/− T cells were positively selected from the spleens of DO11.10 Tg Rag−/− mice by incubating with CD4+ magnetic beads (Miltenyi) and sorting on an Automacs. Each recipient received between 105–106 DO11.10 Rag−/− T cells, by i.v. injection on day 0 as indicated in figure legend. Mice then received three intranasal doses of LPS-free OVA on days 0, 1, and 2. As indicated in Figure legends, DO11.10 Rag−/− T cells were labelled with 5 μM Cell Tracker Green (CMFDA, Life Technologies) or 2 μM CFSE (Sigma). Labelling with Cell Tracker Green was performed according to the manufacturer’s instructions. Briefly, cells are incubated at 37C in complete DMEM with 5 μM CMFDA for 30 min. The cells were then washed and incubated in complete DMEM for 30 min, washed and resusupended in PBS. Labelling with CFSE was performed in PBS for 3 min at room temperature followed by quenching with 40% FCS/PBS. Cells were washed and resuspended in PBS for adoptive transfer.
FACS antibodies and analysis. Mice were treated with three intranasal doses of saline or 100 μg LPS-free OVA. One day later, draining bronchial and mediastinal lymph nodes were removed and collagenase digested. Single cell suspensions were stained at 4C in 0.5% BSA, 2 mM EDTA, PBS. To stain for dendritic cells the following antibody cocktail was used, CD11c APC-A750, MHCII PE, CD103 FITC, CD11b A700, rIgG1 APC or TIM-4 APC, Gr1 PE-Cy7, B220 biotin streptavidin-PE-TexasRed. Macrophages were stained using F4/80 FITC, CD11b A700, rIgG1 APC or TIM-4 APC, and CD11c-APC-A750. Eosinophils were stained using Siglec-F PE and identified by gating on FSC low/SSC intermediate cells. Mice that received DO11.10 T cells were stained for KJ1-26 PE, CD25 PerCP-Cy5.5, TCRβ APC, and CD4 APC-A750. Data was collected on a FACSCanto and data was analyzed using FlowJo software (Tree Star).
Immunofluorescence microscopy staining for TIM-4. Lung draining lymph nodes were mounted in OCT and 6–8 μm sections were cut and affixed to glass slides. Sections were fixed in acetone. For secondary detection of TIM-4, slides were blocked with 10% goat serum and stained with anti-TIM-4 or isotype control at 10 μg/ml in 2% goat serum. Secondary detection used goat anti-rat Cy3 in 2% goat serum for 1 h. For primary detection, slides were blocked with 10 μg/ml Fc block, 10% rat serum, 2.5% BSA. Slides were then stained with anti-TIM-4 Alexa568, CD11c, F4/80, or LYVE-1 Alexa647 in 2% rat serum, 0.5% BSA. Invitrogen Gold anti-fade plus DAPI was used as a mounting media. Coverslips (#1) were affixed to slides using nail polish. Images were collected with a spinning disc confocal microscope with the following oil objectives (numerical aperture): 40 × (1.2), 60 × (1.3), or 100 × (1.4). Confocal images were acquired using a TE-2000 inverted microscope (Nikon Eclipse) with attached Orca AG camera (Hamamatsu Photonics, Hamatsu, Japan). Image collection, linear scaling, and analysis were performed using SlideBook software. Image dimensions and resolution were then adjusted for publication using Adobe Photoshop.
Phagocytosis assay. Preparation of apoptotic cells and assays for engulfment of apoptotic cells were performed essentially as described.6, 10 Thymocytes isolated from 4–5 week old WT BALB/c mice were incubated with 10 μM dexamethasone (Sigma-Aldrich) in RPMI 1640 without FCS for 3 h. Cells were washed and apoptosis was confirmed by Annexin-V-FITC and propidium iodide staining (BD PharMingen). For measurement of phagocytosis, cells isolated from bronchial lymph nodes by collagenase digestion were cultured in cDMEM in 24-well plates for 1 h. Apoptotic thymocytes labeled with pHrodo (1.5 μM, Invitrogen) according to the manufacturers instructions were added to the cells in the ratio of 1:1, 2:1 and 4:1. After two hours of incubation at 37°C, adherent cells were washed twice with PBS and 0.1 mM EDTA and were detached from plates by PBS containing 2 mM EDTA. Cells were then stained for F4/80, CD11b, and TIM-4 and acquired on a FACSCanto.
References
Encinas, J.A. & Kuchroo, V.K. Mapping and identification of autoimmunity genes. Curr. Opin. Immunol. 12, 691–697 (2000).
Marsh, D.G. et al. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science 264, 1152–1156 (1994).
McIntire, J.J., Umetsu, D.T. & DeKruyff, R.H. TIM-1, a novel allergy and asthma susceptibility gene. Springer Semin. Immunopathol. 25, 335–348 (2004).
McIntire, J.J. et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2, 1109–1116 (2001).
Rodriguez-Manzanet, R., DeKruyff, R., Kuchroo, V.K. & Umetsu, D.T. The costimulatory role of TIM molecules. Immunol. Rev. 229, 259–270 (2009).
Kobayashi, N. et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940 (2007).
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Santiago, C. et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-ion-dependent ligand binding site where phosphatidylserine binds. Immunity 27, 941–951 (2007).
Freeman, G.J., Casasnovas, J.M., Umetsu, D.T. & DeKruyff, R.H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 235, 172–189 (2010).
Albacker, L.A. et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J. Immunol. 185, 6839–6849 (2010).
Mizui, M. et al. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int. Immunol. 20, 695–708 (2008).
Rodriguez-Manzanet, R. et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc. Natl. Acad. Sci. USA 107, 8706–8711 (2010).
Akbari, O. et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-Ligand pathway and inhibit allergen-induced airway hyperreactivity. Nature Medicine 8, 1024–1032 (2002).
del Rio, M.L., Rodriguez-Barbosa, J.I., Kremmer, E. & Forster, R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 (2007).
Tsitoura, D.C., DeKruyff, R.H., Lamb, J.R. & Umetsu, D.T. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J. Immunol. 163, 2592–2600 (1999).
Ballesteros-Tato, A., Leon, B., Lund, F.E. & Randall, T.D. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 11, 216–224 (2010).
McComb, S., Mulligan, R. & Sad, S. Caspase-3 is transiently activated without cell death during early antigen driven expansion of CD8(+) T cells in vivo. PLoS One 5, e15328 (2010).
Bedoret, D. et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 119, 3723–3738 (2009).
Akbari, O., DeKruyff, R.H. & Umetsu, D.T. Pulmonary dendritic cells secreting IL-10 mediate T cell tolerance induced by respiratory exposure to antigen. Nature Immunol. 2, 725–731 (2001).
GeurtsvanKessel, C.H. & Lambrecht, B.N. Division of labor between dendritic cell subsets of the lung. Mucosal. Immunol. 1, 442–450 (2008).
Stock, P. et al. Induction of TH1-like regulatory cells that express Foxp3 and protect against airway hyperreactivity. Nature Immunol. 5, 1149–1156 (2004).
Chao, M.P. et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2, 63ra94.
Fischer, K. et al. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood 108, 4094–4101 (2006).
Frasch, S.C. et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J. Biol. Chem. 275, 23065–23073 (2000).
Borisenko, G.G., Iverson, S.L., Ahlberg, S., Kagan, V.E. & Fadeel, B. Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: implications for macrophage clearance of apoptotic cells. Cell Death Differ. 11, 943–945 (2004).
Gonzalez, S.F. et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 11, 427–434 (2010).
Grigorova, I.L. et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 10, 58–65 (2009).
Acknowledgements
We thank Jessica Walters and the Harvard Digestive Diseases Center Imaging Core for expert help and Elena Tonti of the von Andrian lab for training in the removal and orientation of lymph nodes for confocal microscopy. This work was supported by NIH P01 AI054456 (to D.T.U., G.J.F., R.D.K.,), NIH RO1 089955 (to G.F., R.D.K.), and the Program on Immunology, Division of Medical Sciences, Harvard University (to L.A.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Albacker, L., Yu, S., Bedoret, D. et al. TIM-4, expressed by medullary macrophages, regulates respiratory tolerance by mediating phagocytosis of antigen-specific T cells. Mucosal Immunol 6, 580–590 (2013). https://doi.org/10.1038/mi.2012.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.100
This article is cited by
-
Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly
Nature Immunology (2020)
-
Location, function, and ontogeny of pulmonary macrophages during the steady state
Pflügers Archiv - European Journal of Physiology (2017)
-
TIM-4 promotes the growth of non-small-cell lung cancer in a RGD motif-dependent manner
British Journal of Cancer (2015)
-
CD300b regulates the phagocytosis of apoptotic cells via phosphatidylserine recognition
Cell Death & Differentiation (2014)