Abstract
Female reproductive tract (FRT) epithelial cells protect against potential pathogens and sexually transmitted infections. The purpose of this study was to determine if epithelial cells from the upper FRT secrete antimicrobials that inhibit reproductive tract pathogens that threaten women's health. Apical secretions from primary cultures of Fallopian tube, uterine, cervical, and ectocervical epithelial cells were incubated with Neisseria gonorrhoeae, Candida albicans (yeast and hyphal forms), human immunodeficiency virus 1 (HIV-1), and Lactobacillus crispatus before being tested for their ability to grow and/or infect target cells. Epithelial cell secretions from the upper FRT inhibit N. gonorrhoeae and both forms of Candida, as well as reduce HIV-1 (R5) infection of target cells. In contrast, none had an inhibitory effect on L. crispatus. An analysis of cytokines and chemokines in uterine secretions revealed several molecules that could account for pathogen inhibition. These findings provide definitive evidence for the critical role of epithelial cells in protecting the FRT from infections, without comprising the beneficial presence of L. crispatus, which is part of the normal vaginal microflora of humans.
Similar content being viewed by others
Introduction
The mucosal immune system in the reproductive tract and at other mucosal surfaces is the first line of defense against pathogenic organisms.1 The female reproductive tract (FRT) has unique requirements for regulation of immune protection, as it must deal with sexually transmitted pathogens, allogeneic spermatozoa, and the immunologically distinct fetus.1, 2 To meet these challenges, the FRT has evolved unique immune mechanisms to protect against potential pathogens without compromising fetal survival or maternal health. The task is formidable in that >20 pathogens are transmissible through sexual intercourse. Each year, there are an estimated 340 million new cases of sexually transmitted infections from bacteria (group B streptococcus, Neisseria gonorrhoeae, Chlamydia trachomatis, Treponema pallidum), parasites (Trichomonas vaginalis), and viruses (herpes simplex, human papilloma, human immunodeficiency).3 In addition, the yeast Candida albicans is responsible for 85–90% of cases of vulvovaginal candidiasis, which will affect 75% of all women at least once during their lifetime, and others will suffer from recurrent infections.4 C. albicans is described as a commensal microbe in the vagina because of its presence in up to 20% of healthy, asymptomatic women.4, 5 The recognition that commensals and pathogens, including human immunodeficiency virus (HIV), traverse the entire FRT is an essential prerequisite to identifying solutions to preventing and controlling the spread of FRT infections that compromise reproductive health and threaten the lives of women and children.6
Epithelial cells are vital for protection against pathogens in the FRT.7, 8 For example, in addition to providing a physical barrier, FRT epithelial cells possess intracellular and extracellular pathogen recognition receptors (TLR (Toll-like receptor), NOD (nucleotide-oligomerization domain), RIG (retinoic-acid-inducible protein), MDA-5 (melanoma differentiation-associated gene 5), and so on),9, 10, 11 secrete chemokines and cytokines that initiate, regulate, and link innate and adaptive immune responses,12, 13, 14 present antigen to T cells,15, 16 produce the polymeric immunoglobulin receptor for transporting mucosal IgA antibodies from tissues into luminal secretions,17, 18 and produce intracellular and secreted antimicrobial factors that kill invading microbes.19, 20, 21, 22 Among the epithelial cell secretions, molecules with known bactericidal effects are defensins, secretory leukocyte protease inhibitor (SLPI), lysozyme, and numerous other small peptides (for review, see ref. 23). Human β-defensins 1–4 (HBD1–4) and SLPI are effective at killing Gram-positive and Gram-negative bacteria, fungi, and enveloped viruses, including the sexually transmitted infections N. gonorrhoeae, C. trachomatis, herpes simplex virus-2, and HIV-1, as well as C. albicans.19, 24, 25, 26, 27, 28, 29, 30 Other factors with antiviral activity, notably against HIV-1, are the chemokines CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, and CXCL12/SDF-1α,31, 32 which inhibit HIV-1 infection of target host cells by interfering with the ability of HIV-1 to bind co-receptors CCR5 (chemokine (C-C motif) receptor 5) and CXCR4 (chemokine (C-X-C motif) receptor 4) found on host cells. HBD-1 expression has been shown in situ33 and in cultured epithelial cells.11, 21 We showed that TLR3 stimulation of uterine epithelial cells induces the mRNA expression of the antimicrobial peptides HBD1 and HBD2.11 More recently, we demonstrated that human uterine and Fallopian tube epithelial cells produce macrophage inflammatory protein 3α (MIP3α)34 and Trappin-2/Elafin,35 and that these recombinant peptides inhibit HIV infection in a dose-dependent manner. One reason that the endometrium maintains a predominantly sterile environment might be because of the secretion of antimicrobial factors such as defensins, MIP3α, and SLPI.
In the lower FRT, commensal microorganisms such as Lactobacillus provide an acid environment and secrete antipathogenic factors called bacteriocins.36 Commensals are essential to maintaining healthy tissue; removal of commensals through oral intake of antibiotics can lead to pathogen invasion. Factors in FRT secretions that kill pathogens might also adversely affect commensals. Therefore, as a part of these studies, we tested the effect of FRT epithelial cell secretions on Lactobacillus as well as selected potential pathogens.
The purpose of this study was to determine if primary cultures of normal human epithelial cells from throughout the FRT constitutively secrete antimicrobials that inhibit a variety of types of pathogens (bacterium, virus) as well as two commensals. We demonstrate that epithelial cell secretions from Fallopian tube, uterus, endocervix, and ectocervix inhibit the growth and/or infection by N. gonorrhoeae and HIV-1 as well as both the yeast and hyphae forms of C. albicans without affecting Lactobacillus crispatus.
Results
Inhibition of C. albicans (yeast and hyphal forms) growth by human FRT epithelial cell secretions in culture
We have found that human Fallopian tube, uterine, and endocervical columnar epithelial cells, isolated from patients as cell sheets and cultured on cell inserts as previously described,12, 37 attain high values of TER and are functionally polarized. Previously, we showed that apical secretions from uterine epithelial cells cultured in antibiotic- and serum-free media inhibit both Staphylococcus aureus and Escherichia coli replication.19 To determine if uterine apical secretions are capable of inhibiting Candida, yeast and hyphal forms were co-incubated with an aliquot of conditioned media derived from polarized primary uterine epithelial cells. Figure 1a shows that the apical secretions from these cells inhibited C. albicans colony-forming units (CFUs) by >80%. In similar studies using endocervix epithelial cells as well as squamous ectocervical epithelial cells, inhibition of C. albicans was ⩾70% with conditioned media from each of these preparations (Figure 1b). These data indicate that FRT epithelial cells secrete a soluble factor(s) that inhibits both the commensal and pathogenic forms of C. albicans.
The effect of apical secretions from human primary female reproductive tract (FRT) epithelial cells on growth of yeast and hyphal forms of Candida albicans. Conditioned media from (a) uterine and (b) cervix and ectocervix epithelial cells were diluted 1:1 with fresh media and incubated with either the yeast or hyphal forms of C. albicans for 1.5 h before spreading the mixture on plates to enumerate colony-forming units (CFUs) after 24 or 48 h of incubation. Before collection of apical secretions or control media, cells were washed 3 × and incubated for at least 72 h in antibiotic- and serum-free media with two media changes to ensure elimination of antibiotic and serum. The effect of apical secretions on mean CFU±s.e.m. is shown. Control refers to media that had been incubated in cell inserts without cells, diluted 1:1 with fresh media, mixed with the appropriate form of C. albicans preparation, and plated for CFUs. Additional controls were C. albicans mixed directly with fresh media; there was no difference in CFUs between either of these controls. Data presented in a and b are representative of individual tissues (uterus, cervix, and ectocervix) from four patients (epithelial cells grown on 4–6 inserts per patient). **P<0.01 compared with control.
Inhibition of N. gonorrhoeae growth by human FRT epithelial cell secretions in culture
To define more fully the antimicrobial potential of epithelial cell secretions, uterine conditioned media were also incubated with N. gonorrhoeae. As shown in Figure 2a, uterine epithelial cell conditioned media inhibited N. gonorrhoeae growth by ∼47% relative to controls incubated in media alone. Figure 2b illustrates that the conditioned media from Fallopian tube and endocervical cells also significantly inhibited N. gonorrhoeae.
The effect of apical secretions from human primary female reproductive tract (FRT) epithelial cells on growth of Neisseria gonorrhoeae. Conditioned media from (a) uterine and (b) Fallopian tube and endocervix epithelial cells were diluted 1:1 with fresh media and incubated with N. gonorrhoeae for 2 h before spreading the mixture on plates to enumerate colony-forming units (CFUs) after 20 h of incubation. Before collection of apical secretions or control media, cells were washed 3 × and incubated for at least 72 h in antibiotic- and serum-free media with two media changes to ensure elimination of antibiotic and serum. The effect of apical secretions on mean CFU±s.e.m. is shown. Control refers to media that had been incubated in cell inserts without cells, diluted 1:1 with fresh media, mixed with the same N. gonorrhoeae preparation, and plated for CFUs. Additional controls were N. gonorrhoeae mixed directly with fresh media; there was no difference in CFUs between either of these controls. Data presented in a and b are representative of individual tissues (uterus, Fallopian tubes, cervix, and ectocervix) from 3 to 5 patients (4–6 inserts per patient). **P<0.01 compared with control.
Inhibition of HIV-1 infection by secretions from uterine and cervix epithelial cells in culture
To explore the possibility that uterine epithelial cells in culture are capable of inhibiting HIV-1 infection, apical secretions from polarized uterine and cervical epithelial cells were diluted 10-fold and incubated with BaL, an R5/macrophage tropic HIV, respectively. As seen in Figure 3a, diluted uterine apical secretions from epithelial cells from the uteri of two patients significantly inhibited BaL (R5) infection of TZM-bl cells. Similarly, as shown in Figure 3b, when secretions diluted 1:10 from polarized Fallopian tube and cervical epithelial cells as well as confluent ectocervical epithelial cells from the same patient were analyzed, apical secretions inhibited HIV-1 BaL (R5). Conditioned media had no effect on TZM-bl viability (data not shown). Overall, these findings indicate that epithelial cells throughout the human FRT are capable of secreting molecules with anti-HIV activity.
The effect of apical epithelial cell secretions on human immunodeficiency virus 1 (HIV-1) infection. Conditioned media (48 h) from polarized (a) uterine and (b) Fallopian tube, cervical, and ectocervical epithelial cells were diluted 1:10 with fresh media and incubated with BaL HIV-1 for 1 h before addition to TZM-bl cells to assess infection as reported in Methods. Results are compared with infection data obtained with each virus incubated with media alone. Data presented in a and b are representative of individual tissues (uterus, Fallopian tubes, cervix, and ectocervix) from 3 to 7 patients (4–6 inserts per patient). **P<0.01 compared with control.
Lack of an inhibitory effect of FRT epithelial cell secretions on the commensal L. crispatus
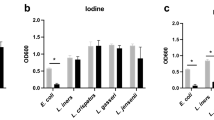
To determine if FRT epithelial cell conditioned media inhibited replication/viability of L. crispatus as they had for C. albicans and N. gonorrhoeae (Figures 1 and 2), the L. crispatus was briefly incubated with various 48 h secretions from FRT epithelial cells. A direct comparison of the same uterine epithelial cell secretion on CFU growth of C. albicans, N. gonorrhoeae, and L. crispatus is shown in Figure 4. In contrast to the inhibition of C. albicans and N. gonorrhoeae, we found no evidence of inhibition by conditioned media of L. crispatus. As seen in Figure 5, when apical secretions from epithelial cells isolated from the Fallopian tubes, uterus, endocervix, and ectocervix were analyzed, no differences in L. crispatus CFUs between any of the secretions and the control were detected.
Comparison of antimicrobial activity against Neisseria gonorrhoeae, Candida albicans, and Lactobacillus crispatus. Results shown are the percent inhibition obtained with uterine apical conditioned media derived from four cell inserts of one patient. For each microorganism, the colony-forming unit (CFU) obtained with conditioned media incubation was compared with four controls of the microorganism incubated with media. **P<0.01 compared with control.
The effect of apical secretions from human primary female reproductive tract (FRT) epithelial cells on growth of Lactobacillus crispatus. Conditioned media from Fallopian tube, uterine, endocervix, and ectocervix epithelial cells were diluted 1:1 with fresh media and incubated with L. crispatus for 1 h as noted in Methods before spreading the mixture on plates to enumerate colony-forming units (CFUs) after 48 h of incubation. Before collection of apical secretions or control media, cells were washed 3 × and incubated for at least 72 h in antibiotic- and serum-free media with two media changes to ensure elimination of antibiotic and serum. The effect of apical secretions on mean CFU±s.e.m. is shown. Control refers to media that had been incubated in cell inserts without cells, diluted 1:1 with fresh media, mixed with the same L. crispatus preparation, and plated for CFUs. Additional controls were L. crispatus mixed directly with fresh media; there was no difference in CFUs between either of these controls. Data shown are the mean CFU±s.e.m. from 3 to 4 cell inserts from epithelial cells derived from a total of seven designated patients.
Candidate antimicrobials in uterine epithelial cell secretions
Primary uterine apical epithelial cell secretions were analyzed by enzyme-linked immunosorbent assay and Luminex bead analysis to identify some of the candidate antimicrobial molecules. As seen in Table 1, a number of chemokines, which are often directly antimicrobial to bacteria, were found at concentrations capable of inhibiting microbes. In addition, several molecules that are known to inhibit HIV-1 infection were also present. For example, RANTES (regulated upon activation, normal T cell expressed and secreted), SDF-1 (stromal cell-derived factor-1), SLPI, MIP3α, MIP1α, MIP1β, HBD2, and Trappin-2/Elafin have all been shown to block HIV-1 infection, either by binding to HIV co-receptors, direct inactivation of the virus, or other mechanisms not yet defined.34, 35, 38, 39, 40 These factors, also found at concentrations capable of inhibiting HIV-1, might act individually or in synergy to inactivate pathogens.
Discussion
Mucosal surfaces are the first line of defense against potentially pathogenic microorganisms. Mucosal FRT epithelial cells have the capacity to recognize and respond immediately to pathogens and can rapidly block infection from being established. In a previous study,19 we demonstrated that human uterine epithelial cells in culture produce an antibacterial factor(s) that is equally effective in inhibiting the growth of a Gram-positive (S. aureus) and a Gram-negative bacterium (E. coli). To explore the possibility that secretions from epithelial cells along the entire FRT have antimicrobial activity, cultured epithelial cells from the Fallopian tubes, uterus, endocervix, and ectocervix were grown to confluence in cell inserts, and assessed for their ability to secrete a substance(s) into the apical compartment that would inhibit bacterial and fungal growth, as well as HIV-1 infectivity. We found that epithelial cell conditioned media from all FRT sites examined inhibited growth of C. albicans (yeast and hyphae) and N. gonorrhoeae. In addition, FRT epithelial secretions were effective in significantly reducing HIV infection of TZM-bl cells. Just as important, FRT epithelial cell secretions had no effect on the growth of the urogenital commensal L. crispatus. Although vaginal squamous cells were not available owing to the hysterectomy specimens provided, squamous cells from ectocervical tissues were included in this study. As seen in Figures 1, 3, and 5, secretions from these cells had inhibitory activities comparable with that seen with columnar epithelial cells from the upper FRT.
It is well recognized that C. albicans is both a commensal and a pathogen in the lower FRT. As a part of its life cycle, C. albicans exists in a nonpathogenic yeast form as well as a potential pathogen during the hyphal stage. Recognized as a common component of the digestive and genital floras, C. albicans has the potential to cause superficial as well as disseminated infections in response to host immune system changes, oral contraceptive use, or microflora alterations.4, 41 In contrast to the vagina, the upper FRT lacks C. albicans receptors and an appropriate nutritional status that most likely confer vaginal tissue tropism. Our studies demonstrate that FRT epithelial cells secrete a spectrum of antimicrobials that have anti-C. albicans (yeast and hyphal forms) activity. An unexpected finding in our study was the differential effect of epithelial cell secretions on the commensal form of C. albicans (yeast) and Lactobacillus. Whereas all secretions tested inhibited the commensal form of C. albicans, none had an inhibitory effect on Lactobacillus. What is clear is that factors in epithelial secretions selectively discriminate between these two commensals.
Analysis of conditioned media led to the identification of a spectrum of antimicrobials, chemokines, and cytokines secreted by uterine epithelial cells. We have analyzed the conditioned media for biological activity and identified MCP-1 (monocyte chemotactic protein-1), IL-8 (interleukin-8), GM-CSF (granulocyte-macrophage colony-stimulating factor), and SLPI based on either chemotaxis or antimicrobial assays.13, 19, 42 Many of these innate immune factors are multifunctional in that they exhibit both chemokine and antimicrobial activity. For example, MIP3α is known to be chemotactic for immature dendritic cells, T cells, and B cells.43, 44 This chemokine was found to inhibit both Gram-negative and Gram-positive bacteria.45 Our recent studies extend these findings by demonstrating that MIP3α as well as Trappin-2/Elafin are potent antiviral molecules capable of blocking HIV-1 infection of target cells.34, 35 The extent to which other molecules secreted by epithelial cells throughout the FRT have cytokine and chemokine activity, as well as potent antibacterial, antifungal, and antiviral activity, remains to be determined.
In other studies in which we identified rElafin and rCCL20/MIP3α as novel antimicrobials against HIV,34, 35 we found that neutralization of recombinants was possible with complete reversal of anti-HIV-1 activity. However, using these same antibodies, we did not reverse the anti-HIV-1 activity in uterine apical secretions containing Elafin and MIP3α, present at concentrations that should be completely blocked. We and others have previously shown that FRT secretions contain a family of antimicrobials, which have anti-HIV-1 activity.34, 35 As Elafin and MIP3α are two of the many molecules present in these secretions, neutralization would not be measurable as a separate entity.
To the best of our knowledge, this is the first study to demonstrate that epithelial cells from throughout the human FRT, under constitutive conditions, inhibit a wide range of organisms that both compromise women's reproductive health and are potentially life threatening. Others have shown that commensal microorganisms are constitutively present in the lower FRT as part of a genital tract ecosystem that prevents the establishment of infections by pathogens via a variety of mechanisms (adherence, pH, bacteriocins, and so on). Our studies suggest that a dynamic balance exists in the upper FRT between innate immune epithelial cell factors and commensals in maintaining a healthy homeostatic microenvironment in the lower FRT. Unlike antibiotics, which can kill commensal bacteria in the lower FRT and thereby lead to opportunistic infections, endogenous epithelial factors are constitutively produced to provide long-term protection against pathogens. The mechanism(s) by which Lactobacillus is resistant to endogenous antimicrobials remains elusive, and is further complicated by the recent discovery that Lactobacillus stimulates β-defensin secretion by enterocytes.46 Commensals and epithelial cells act separately as well as in conjunction to protect the FRT from infection. This symbiotic relationship is particularly appropriate when one considers that secretions from the upper tract flow to the lower tract, whereas lower tract secretions, as well as sperm, move into the upper tract.47
What remains to be determined is how genital tract secretions exert their inhibitory effects on bacterial, fungal, and viral pathogens. Antimicrobials in the defensin family have been shown to cause pore formation in bacterial cell walls, resulting in death of the bacterium. RANTES blocks HIV-1 infection by binding to the HIV co-receptor CCR5, preventing viral attachment, whereas Trappin-2/Elafin and MIP3α inhibit HIV-1 infection by interacting with the virus.34, 35 SLPI, which inhibits Candida,29, 48 HIV,26, 30, 49, 50 and bacteria,51 has multiple mechanisms to protect the FRT. For example, SLPI inhibits HIV-1 infection by binding to annexin-1 on the cell surface, and by inhibiting NF-κB stimulation for immune activation that promotes HIV infection. CCL28 inhibits bacteria and Candida52 and possibly HIV infection by binding to CCR3, which has been shown to be an alternative co-receptor for HIV-1.53 Regardless of mechanism, it is clear that epithelial cells secrete a spectrum of antimicrobials that are protective against microbial pathogens.
Recognizing that sexual transmission is almost exclusively initiated by HIV-1 variants with tropism for the CCR5 co-receptor (R5 viruses),54 we tested epithelial secretions and found that Fallopian tube, uterine, cervix, and ectocervix epithelial secretions inhibited BaL (R5) virus. To the best of our knowledge, this is the first demonstration that secretions from epithelial cells throughout the human FRT have anti-HIV activity. Further studies are needed to identify the antimicrobial(s) responsible for inhibition of HIV and whether the antimicrobials secreted are cell and site specific, or produced by epithelial cells independent of their anatomical location (upper vs. lower FRT). In other studies, we have recently found that cervical vaginal secretions from both HIV (−) women and HIV (+) women who are healthy and not on antiretrovirals have anti-HIV activity against R5 HIV-1.55 These findings suggest that antimicrobials produced by epithelial cells from throughout the reproductive tract have an important protective role in vivo against the transmission of HIV from men to women.
In conclusion, these studies demonstrate that normal FRT epithelial cells secrete a spectrum of antimicrobials in culture that have broad-spectrum activity against bacterial, fungal, and viral pathogens. These results emphasize the central role of uterine epithelial cells in protecting the FRT from sexually transmitted diseases and opportunistic infections.
Methods
Source of FRT tissues. FRT mucosal tissues were obtained immediately following surgery from women who had undergone hysterectomies at Dartmouth-Hitchcock Medical Center. Tissues used in this study were distal to the sites of pathology and were determined to be unaffected with disease upon inspection by a trained pathologist. Pathologists also determined the menstrual status, as well as the stage in the cycle of premenopausal patients by examining the endometrial glands for secretory changes. Written informed consent was obtained from patients before surgery. Approval to use tissues was previously obtained from the Committee for the Protection of Human Subjects, in accordance with the human experimentation guidelines of the US Department of Health and Human Services. For cell culture studies, 17 FRT tissues were obtained from 11 women (age range 28–77). This patient population is detailed in Table 2. Of those patients included in the study, two had a post-operative diagnosis of malignant FRT disease. Single 0.75–3 g samples of tissue were dissected out from equivalent sites of the FRT, distal to any gross pathology.
Isolation of FRT epithelial cells. Epithelial cells were isolated as previously described.12, 37 Briefly, tissues were minced under sterile conditions into 1 to 2 mm fragments and subjected to enzymatic digestion using a “PHC” enzyme mixture that contained final concentrations of 3.4 mg ml–1 pancreatin (Invitrogen, Grand Island, NY), 0.1 mg ml–1 hyaluronidase (Worthington Biochemical, Freehold, NJ), 1.6 mg ml–1 collagenase (Worthington), and 2 mg ml–1 D-glucose, in 1 × Hanks’ balanced salt solution (Invitrogen). Enzymes were chosen to maximize digestion of the extracellular matrix while minimizing digestion of cell surface antigens. After incubating for 1 h at 37 °C, cells were dispersed through a 250-micron mesh screen, washed, and resuspended in Dulbecco's modified Eagle's medium/F12 complete medium. Epithelial cell sheets were separated from stromal cells by serial filtration through 40- and 20-micron nylon mesh filters (Small Parts, Miami Lakes, FL). Epithelial sheets were collected, centrifuged at 500 g, and resuspended in complete medium supplemented with 20 mM HEPES, 2 mM L-glutamine (both from Life Technologies, Carlsbad, CA), Primocin (50 μg ml–1, Invivogen, San Diego, CA), and 10% heat-inactivated defined fetal bovine serum (Hyclone, Logan, UT). Using this procedure, we have isolated epithelial cells that stain positive with antibodies for the epithelial antigens Ber-EP4 and cytokeratin and negative for CD4, CD45, and vimentin (Calbiochem, San Diego, CA). The antibody studies and the attainment of high transepithelial resistance for cells derived from the upper reproductive tract indicate that cultures contain purified epithelial cells. Ectocervical epithelial cells, which do not develop transepithelial resistance, were also isolated in this manner and grown out to confluence on the cell inserts.
Cell culture. To establish cell cultures of polarized human Fallopian tube, uterine, and endocervical (cervix) epithelial cells with both apical and basolateral compartments, cells were cultured on human extracellular matrix (BD Biosciences, San Jose, CA)-coated Falcon cell culture inserts (Fisher Scientific, Pittsburgh, PA). Apical and basolateral compartments had 300 and 850 μl of complete medium, respectively. As an indicator of tight junction formation in upper FRT epithelial cell monolayers, transepithelial resistance was assessed daily using an EVOM electrode and Voltohmmeter (World Precision Instruments, Sarasota, FL), as described previously.12, 37 Once high transepithelial resistance (700–2,000 ohms/well) for upper tract epithelial cells or confluence for squamous ectocervical epithelial cells was attained, cells were washed and incubated for at least 4 days in serum- and antibiotic-free media with media changes every 48 h. Removal of serum, which might contain complement and pathogen-specific antibodies, and antibiotics was essential for measuring microorganism growth. Following media changes, cells were cultured in fresh serum- and antibiotic-free media for an additional 48 h, before collection of conditioned media from apical and basolateral chambers. Conditioned medium was centrifuged at 500 g for 5 min and frozen at −80 °C until assayed.
Serial dilutions of conditioned media were carried out that ranged from 1:1 to 1:100. We found that dilutions of 1:1 had no effect on inhibiting Lactobacillus, 1:4 was the highest dilution that significantly inhibited Candida, 1:8 was the highest for N. gonorrhoeae, and 1:10 the highest dilution for HIV. Because secretions from epithelial cells contain a number of antimicrobials, studies were carried at the same dilution (1:1) for Lactobacillus, Candida, and N. gonorrhoeae to compare inhibition between FRT cells. Only HIV analysis was carried out in a 1:10 dilution at a time different from the Lactobacillus, Candida, and N. gonorrhoeae studies.
Measurement of anti-HIV activity: TZM-bl assay for HIV-1 Infection. The TZM-bl indicator cell line is a HeLa cell derivative that expresses high levels of CD4 and CCR5 and CXCR4.56 TZM-bl cells were maintained in TZM media consisting of Dulbecco's modified Eagle's medium supplemented with 10% defined fetal bovine serum, 2 mM L-glutamine, and 50 μg ml–1 Primocin. TZM-bl cells were seeded at 2 × 104 cells per well in a 96-well microtiter plate and allowed to adhere overnight at 37 °C. Apical secretions were diluted 1:10 and incubated with BaL (R5 tropic; provided by Dr Phalguni Gupta, University of Pittsburgh, Pittsburgh, PA) at a multiplicity of infection of 1 for 1 h at 37 °C in a final volume of 100 μl. Following incubation, the media was aspirated from TZM-bl cells and the virus plus secretion mixture added to the cells along with 100 μl of TZM-bl media. Briefly, the supernatants were aspirated at 48 h, and the cells were lysed with a β-Glo luciferase assay substrate (Promega, Madison, WI). Uninfected cells were used to determine background luminescence. All infectivity assays were performed in quadruplicate.
Analysis of N. gonorrhoeae inhibition. N. gonorrhoeae (ATCC 31426, Manassas, VA) was streaked on a chocolate agar plate (Remel, Lenexa, KS) and incubated for 18–20 h at 37 °C in 5% CO2 in a humidified incubator. From this plate, the N. gonorrhoeae colony was inoculated into prewarmed GC broth and incubated for 4 h at 37 °C, after which absorbance at 600 nm was measured. Cultures were adjusted with sterile phosphate-buffered saline (PBS) to ∼1 × 106 CFU per ml. A total of 10 μl of culture was added to 100 μl media, or FRT epithelial cell secretions serially diluted in sterile PBS. After 2 h at 37 °C, suspensions were serially diluted in sterile PBS, plated to chocolate agar, and incubated overnight for 20 h. CFUs were counted and percent inhibition calculated.
Assay for effect of secretions on yeast and hyphal C. albicans growth. One colony of the clinical/virulent strain V9:0327:TK of C. albicans was used to inoculate synthetic dextrose minimal medium and incubated for 48 h to reach stationary phase with shaking at 250r.p.m. Cells were washed twice in sterile PBS and suspended at a concentration of 2,500 cells ml–1 based on hemacytometer counting. The washed yeast suspension (40 μl) was incubated for 1.5 h with an equal volume of undiluted conditioned media from human epithelial cells in 0.5 ml microcentrifuge tubes at 30 °C to test the effect on yeast growth. Contents were transferred to yeast peptone dextrose agar plates, spread over the surface using a sterile microbiological spreader, and incubated overnight at 30 °C. CFUs were counted at 24 h. The assay was performed in triplicate. Conditions were similar for assessing the effect of conditioned media on hyphal growth except that cells were resuspended in M199 prewarmed at 37 °C and the conditioned media was also prewarmed to 37 °C. Growth in yeast or hyphal morphology was verified microscopically using controls at a concentration of 4 × 106 cells per ml at 30 or 37 °C, respectively.
Assay for measuring the effect of FRT secretions on L. crispatus. L. crispatus (ATCC 33197) was inoculated from a glycerol stock into 4 ml of Mann Rogosa Sharpe broth and cultured overnight at 37 °C under anaerobic conditions without supplemental CO2. The culture was centrifuged, and the bacterial pellet resuspended in 3 ml of PBS to an OD600=0.2. The bacteria were then serially diluted tenfold to a final concentration of ∼1 × 104 CFU per /ml. In all, 20 μl of the bacterial suspension was added to an equal volume of culture supernatants from the FRT cells, or media controls. The mixture was incubated for 1 h at 37 °C. PBS (60 μl) was then added to each tube, and the bacteria plated immediately on Mann Rogosa Sharpe agar plates. The plates were incubated overnight at 37 °C in an anaerobic chamber and the number of bacterial colonies enumerated. Data are expressed as the mean± s.d. of bacterial colonies on replicate plates.
Analysis of antimicrobials in epithelial cell secretions. Epithelial cell secretions were assayed for 30 different antimicrobials, chemokines, and cytokines by either enzyme-linked immunosorbent assay (R&D, Minneapolis, MN; and Peprotech, Rocky Hill, NJ) or Luminex bead analysis (Bio-Rad, Hercules, CA), as previously described.12 A minimum of four cell inserts was used from each epithelial cell source.
Statistical analysis. A two-tailed paired t-test or a one-way analysis of variance with Bonferonni's post-test was performed using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA). A P-value of <0.05 was taken as indicative of statistical significance.
References
Wira, C.R., Fahey, J.V., Sentman, C.L., Pioli, P.A. & Shen, L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005).
Kutteh, W.H., Mestecky, J. & Wira, C.R. Mucosal immunity in the human female reproductive tract. In Mucosal Immunology (Mestecky, J. et al., eds) 1631–1647 ( Academic Press, New York, 2005 ).
WHO. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates, Geneva, Switzerland, (2005).
Fidel, P.L. Jr History and update on host defense against vaginal candidiasis. Am. J. Reprod. Immunol. 57, 2–12 (2007).
Marot-Leblond, A. et al. Efficient diagnosis of vulvovaginal candidiasis by use of a new rapid immunochromatography test. J. Clin. Microbiol. 47, 3821–3825 (2009).
Wira, C.R. & Fahey, J.V. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids 22, 1909–1917 (2008).
Wira, C.R. & Fahey, J.V. The innate immune system: gatekeeper to the female reproductive tract. Immunology 111, 13–15 (2004).
Wira, C.R., Grant-Tschudy, K.S. & Crane-Godreau, M.A. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am. J. Reprod. Immunol. 53, 65–76 (2005).
Pioli, P.A. et al. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect. Immun. 72, 5799–5806 (2004).
Schaefer, T.M., Desouza, K., Fahey, J.V., Beagley, K.W. & Wira, C.R. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112, 428–436 (2004).
Schaefer, T.M., Fahey, J.V., Wright, J.A. & Wira, C.R. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J. Immunol. 174, 992–1002 (2005).
Fahey, J.V., Schaefer, T.M., Channon, J.Y. & Wira, C.R. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum. Reprod. 20, 1439–1446 (2005).
Meter, R.A., Wira, C.R. & Fahey, J.V. Secretion of monocyte chemotactic protein-1 by human uterine epithelium directs monocyte migration in culture. Fertil. Steril. 84, 191–201 (2005).
Schaefer, T.M., Fahey, J.V., Wright, J.A. & Wira, C.R. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C). Am. J. Reprod. Immunol. 54, 193–202 (2005).
Fahey, J.V., Wallace, P.K., Johnson, K., Guyre, P.M. & Wira, C.R. Antigen presentation by human uterine epithelial cells to autologous T cells. Am. J. Reprod. Immunol. 55, 1–11 (2006).
Wallace, P.K. et al. MHC class II expression and antigen presentation by human endometrial cells. J. Steroid. Biochem. Mol. Biol. 76, 203–211 (2001).
Fahey, J.V., Humphrey, S.L., Stern, J.E. & Wira, C.R. Secretory component production by polarized epithelial cells from the human female reproductive tract. Immunol. Invest. 27, 167–180 (1998).
Wira, C., Fahey, J., Wallace, P. & Yeaman, G. Effect of the menstrual cycle on immunological parameters in the human female reproductive tract. J. Acquir. Immune. Defic. Syndr. 38 (Suppl 1), S34–S36 (2005).
Fahey, J.V. & Wira, C.R. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J. Infect. Dis. 185, 1606–1613 (2002).
King, A.E., Critchley, H.O. & Kelly, R.W. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol. Hum. Reprod. 6, 191–196 (2000).
King, A.E., Fleming, D.C., Critchley, H.O. & Kelly, R.W. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol. Hum. Reprod. 8, 341–349 (2002).
King, A.E., Fleming, D.C., Critchley, H.O. & Kelly, R.W. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J. Reprod. Immunol. 59, 1–16 (2003).
Ganz, T. & Lehrer, R.I. Defensins. Pharmacol. Ther. 66, 191–205 (1995).
Duits, L.A. et al. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol. Med. Microbiol. 38, 59–64 (2003).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Hocini, H. et al. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin. Diagn. Lab Immunol. 7, 515–518 (2000).
Porter, E. et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect. Immun. 73, 4823–4833 (2005).
Quinones-Mateu, M.E. et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids 17, F39–48 (2003).
Tomee, J.F., Hiemstra, P.S., Heinzel-Wieland, R. & Kauffman, H.F. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J. Infect. Dis. 176, 740–747 (1997).
Wahl, S. et al. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-1. Oral Dis. 3 (Suppl 1), S64–S69 (1997).
Bleul, C.C. et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833 (1996).
Cocchi, F. et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270, 1811–1815 (1995).
Valore, E.V. et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest. 101, 1633–1642 (1998).
Ghosh, M. et al. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am. J. Reprod. Immunol. 62, 60–71 (2009).
Ghosh, M. et al. Trappin-2/Elafin: a novel innate anti-HIV-1 molecule of the human female reproductive tract. Immunology 129, 207–219 (2009).
Martin, R. et al. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int. Microbiol. 11, 261–266 (2008).
Fahey, J.V., Kaushic, C. & Wira, C.R. Human uterine epithelial cells: influence of culture conditions and stromal cells on epithelial cell transepithelial cell resistance. In Reproductive Immunology (Gupta, S.K., ed) 366–378 ( Narosa Publishing House, New Delhi, 1999 ).
Bingle, C.D. & Vyakarnam, A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 29, 444–453 (2008).
Garzino-Demo, A. Chemokines and defensins as HIV suppressive factors: an evolving story. Curr. Pharm. Des. 13, 163–172 (2007).
Schols, D. HIV co-receptors as targets for antiviral therapy. Curr. Top. Med. Chem. 4, 883–893 (2004).
Calderone, R. Taxonomy and biology of Candida. In Candida and Candidiasis (Calderone, R., ed) 307–325 ( ASM Press, Washington, DC, 2002 ).
Shen, L. et al. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 230, 23–32 (2004).
Thomachot, M.C. et al. Breast carcinoma cells promote the differentiation of CD34+ progenitors towards 2 different subpopulations of dendritic cells with CD1a(high)CD86(−)Langerin- and CD1a(+)CD86(+)Langerin+ phenotypes. Int. J. Cancer 110, 710–720 (2004).
Perez, O.D., Mitchell, D. & Nolan, G.P. Differential role of ICAM ligands in determination of human memory T cell differentiation. BMC Immunol. 8, 2 (2007).
Crane-Godreau, M.A. & Wira, C.R. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infect. Immun. 73, 4231–4237 (2005).
Schlee, M. et al. Probiotic lactobacilli and VSL. Clin. Exp. Immunol. 151, 528–535 (2008).
Zervomanolakis, I. et al. Physiology of upward transport in the human female genital tract. Ann. NY Acad. Sci. 1101, 1–20 (2007).
Chattopadhyay, A. et al. Salivary secretory leukocyte protease inhibitor and oral candidiasis in human immunodeficiency virus type 1-infected persons. Infect. Immun. 72, 1956–1963 (2004).
Py, B. et al. The phospholipid scramblases 1 and 4 are cellular receptors for the secretory leukocyte protease inhibitor and interact with CD4 at the plasma membrane. PLoS One 4, e5006 (2009).
Shugars, D. Endogenous mucosal antiviral factors of the oral cavity. J. Infect. Dis. 179 (Suppl 3), S431–S435 (1999).
Hiemstra, P.S. et al. Antibacterial activity of antileukoprotease. Infect. Immun. 64, 4520–4524 (1996).
Hieshima, K. et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 170, 1452–1461 (2003).
Aasa-Chapman, M.M., Seymour, C.R., Williams, I. & McKnight, A. Novel envelope determinants for CCR3 use by human immunodeficiency virus. J. Virol. 80, 10884–10889 (2006).
Margolis, L. & Shattock, R. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat. Rev. Microbiol. 4, 312–317 (2006).
Ghosh, M. et al. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels with IgA and IgG antibodies. PLoS One 5, e11366 (2010).
Steele, C. & Fidel, P.L. Jr Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 70, 577–583 (2002).
Acknowledgements
We thank Richard Rossoll (Dartmouth Medical School) and Deena Ratner (University of Pittsburgh) for excellent technical assistance in the preparation of samples, cells, and virus stocks. Additionally, we thank Vincent Memoli, Section Chief of Anatomical Pathology, for procuring tissues; and other members of the Department of Pathology for inspecting and dissecting tissue specimens: Jorge Gonzalez, Alan Schned, Peter Seery, Shannon Schutz, Elizabeth Rizzo, Richard Merrill, Charles-Robert Moultry, Patricia Larkin, Aimee Larson, Jennifer Simonton, and Dawn Maddaline; for clinical support and scheduling: Laura Wolfe, Linda Hallock, Kathleen Pilchman, Karen Carter, Kris Ramsey, Tamara Krivit, and Joanne Lavin; surgeons: Barry Smith, Joan Barthold, Jackson Beecham, John Currie, Leslie Demars, Paul Hanissian, John Ketterer, Benjamin Mahlab, Paul Manganiello, Misty Porter, Karen George, William Young, Kris Strohbehn, Roger Young, Stephen Andrews, and Eric Sailer; OR nurses: Jeanette Sawyer, Tracy Stokes, Fran Reinfrank, and Jaclyn Logan. This work was supported by AI51877 and AI071761 (awarded to Dr Charles Wira) from the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Wira, C., Ghosh, M., Smith, J. et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol 4, 335–342 (2011). https://doi.org/10.1038/mi.2010.72
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.72