Abstract
Intestinal CD4+ T cells are rapidly and profoundly depleted in human immunodeficiency virus (HIV)-infected patients and simian immunodeficiency virus (SIV)-infected macaques. However, monitoring intestinal cells in humans is difficult, and identifying surrogate markers in the blood, which correlate with loss or restoration of intestinal CD4+ T cells could be helpful in monitoring the success of therapeutic strategies and vaccine candidates. Recent studies indicate HIV utilizes the intestinal homing molecule α4β7 for attachment and signaling of CD4+ T cells, suggesting this molecule may have a central role in HIV pathogenesis. Here, we compared β7HIGH integrin expression on CD4+ T cells in blood with loss of CD4+ T cells in the intestine of macaques throughout SIV infection. The loss of β7HIGH CD4+ T cells in blood closely paralleled the loss of intestinal CD4+ T cells, and proved to be a more reliable marker of intestinal CD4+ T-cell loss than monitoring CCR5+ memory CD4+ T cells. These data are consistent with a recent hypothesis that α4β7 has a role in the selective depletion of intestinal CD4+ T cells, and indicate that monitoring β7HIGH expression on CD4+ T cells in the blood may be a useful surrogate for estimating intestinal CD4+ T cell loss and restoration in HIV-infected patients.
Similar content being viewed by others
Introduction
The intestinal tract is known to be a major site of early human immunodeficiency virus (HIV) infection and replication, and is likely a major reservoir for viral persistence, particularly in patients on anti-retroviral therapy.1, 2 Thus, a number of investigators are sampling intestinal CD4+ T cells to monitor response to anti-retroviral therapy.1, 3, 4 Furthermore, understanding the dynamics of HIV infection and replication, specifically in mucosal tissues, is fundamental to understanding the pathogenesis of HIV infection, and probably to the development of an effective HIV-1 vaccine.5 For these and other reasons, there is an emerging consensus that measuring intestinal mucosal immune responses may be important for measuring the effectiveness of HIV-1 vaccines and treatment strategies.
However, examining intestinal tissues in humans is problematic, and not without risk. Intestinal biopsies require specialized equipment and skills, and it is unlikely that they can be routinely performed in the field, especially in the context of a large vaccine trial. Therefore, it would be very useful to identify a surrogate marker for tracking changes in mucosal CD4+ T cells which could be routinely measured in blood to predict responses to therapy, vaccines, or progression to AIDS.
The integrin α4β7 mediates lymphocyte migration to the intestine through interaction with the mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is predominantly expressed on venules in the gut-associated lymphoid tissue (GALT) and intestinal lamina propria.6, 7 Thus, cells expressing α4β7 in the blood are believed to traffic predominantly to the intestine. Recently, HIV-1 gp120 was shown to bind and signal through α4β7, which provides an additional explanation for HIV's selective tropism for the intestinal immune system, in addition to the preponderance of CCR5+ memory phenotype CD4+ T cells in this location.8, 9, 10 Furthermore, HIV-1 binding to α4β7 triggers cellular activation and may facilitate selective infection of these cells, or the formation of viral synapses, which may facilitate cell-to-cell HIV-1 transmission.5 We thus hypothesized that the level of circulating α4β7+ CD4+ T cells in blood would correlate with the level of CD4+ T cells remaining in the intestine, and may serve as a surrogate marker for monitoring intestinal CD4+ T-cell depletion or reconstitution in simian immunodeficiency virus (SIV)-infected macaques, and by inference, HIV-infected patients.
Unfortunately, anti-human/non-human primate monoclonal antibodies to α4β7 are limited, and not commercially available. However, cross-reactive antibodies to the integrin β7 are available, and although this detects both αEβ7 (intestinal epithelial) as well as α4β7 (lamina propria) lymphocytes, αEβ7 is rarely expressed in the blood, and its role in homing is less clear.11, 12 We thus used β7 integrin alone, and in combination with CD49d (α4 integrin) to examine and compare mucosal homing lymphocytes.
Results
Distribution of β7HIGHCD4+T cells in tissues of normal rhesus macaques
To confirm that β7HIGH integrin expression was specific for intestinal tissues and cells homing to gut in macaques, the expression and distribution of β7HIGHCD4+ T cells was compared in multiple regions of gut, lymph nodes, spleen, thymus, bone marrow, and peripheral blood of normal rhesus macaques. The majority of β7HIGHCD4+ T cells were intestinal lamina propria lymphocytes in the jejunum, ileum, and colon as well as intestinal intraepithelial lymphocytes. Substantial numbers of β7HIGHCD4+ T cells were also detected in the blood and mesenteric lymph nodes. In contrast, very few (0.7 to 1.5%) β7HIGHCD4+ T cells were detected in the spleen, and β7HIGH expression was practically absent in the thymus and bone marrow (Figure 1). Interestingly, only a fraction (∼15%) of intestinal CD4+ cells were β7HIGH suggesting that this integrin may be downregulated after the cells reach their target tissues, which has been shown for murine CD8+ T cells.13, 14 However, the majority of intestinal cells expressed low or intermediate levels of β7 (not shown). Importantly, few β7HIGHCD4 cells were present in the axillary's LN, despite large numbers being detected in the mesenteric LN (Figure 1). Combined, this validated that β7HIGH expression in macaques was primarily limited to intestines and tissues involved in the gut-homing pathway (mesenteric lymph nodes and blood).
Distribution of β7HIGH integrin expression on CD4+ T cells from various tissues in normal (non-infected) rhesus macaques. Note that the vast majority of β7HIGHCD4+ T cells reside in intestine tissues (IEL and LPL) and in blood. Also note that far more β7HIGHCD4+ T cells are present in the mesenteric lymph node compared with axillary lymph nodes, thymus, or bone marrow. Data represent means ± s.e.m. from five normal macaques examined. IEL, intestinal intraepithelial lymphocytes; LPL, lamina propria lymphocytes.
CD4+T cells expressing β7HIGH have a “memory” phenotype
Both HIV-1 and SIV selectively infect and deplete memory CD4+ T cells in early infection.8, 15, 16, 17, 18 Thus to determine if α4β7HIGH CD4+ T cells express a memory phenotype, we examined the coexpression of CD45RA (a naive cell marker) CD95 (a memory marker) and CD49d (α4 integrin) on CD4+ β7HIGH T cells. In all tissues, β7HIGH CD4+ T cells were essentially all memory cells (CD45RA− and CD95+)(Figure 2). In contrast, β7 “intermediate” CD4+ T cells were all naive cells (CD45RA+, CD95–). In the blood, all + β7HIGH CD4+ cells also coexpressed CD28, consistent with a “central memory” phenotype (data not shown). Furthermore, essentially all β7HIGH CD4+ T cells in the blood coexpressed the α4 integrin (Figure 2). Combined, these results indicated that circulating β7HIGH CD4+ T cells in blood and lymph nodes were all α4β7+ central memory cells, and likely homing to intestinal tissues. Thus, we hypothesized that tracking this cell subset in the blood could predict changes in CD4+ T cells in the intestinal tract.
Phenotyping β7HIGHCD4+ T cells in rhesus macaques. (a) Coexpression of naive (CD45RA) and memory (CD95) markers with β7 HIGH integrin expression on CD4+ T cells from various tissues of representative macaques. Note that although the majority of lymphocytes express low or intermediate levels of β7, essentially all β7HIGHCD4+ T cells (delineated by oval regions) have a memory (CD45RAnegCD95+) phenotype. (b) Gating through β7HIGH CD4+ T cells in two representative animals shows that essentially all β7HIGHCD4+ T cells are also α4HIGH (CD49dHIGH), indicating that almost β7HIGHCD4+ T cells in the blood are α4β7+. All plots were generated by gating through lymphocytes, and then through CD4+ T cells.
Loss of circulating β7HIGH CD4+ T cells parallels the loss of intestinal CD4+ T cells in SIV infection
To determine if changes in β7HIGH CD4+ T cells reflected changes in intestinal CD4+ T cells, we compared cells from the blood and intestine of animals at various stages of SIV infection. As expected, intestinal CD4+ T cells were markedly and persistently depleted within 13 days after infection in all animals examined (Figure 3). Although a mean of 38% of lymphocytes in the intestine were CD4+ before infection, only 5–15% (9±3.2) of the remaining lymphocytes were CD4+ by 13 days of infection (Figure 3). Furthermore, intestinal CD4+ T cells remained depleted in virtually all animals infected for more than 13 days, regardless of the stage of infection (Figure 3). In addition, β7HIGHCD4+ T cells were also rapidly and selectively depleted in the intestine in acute SIV infection (Figures 3 and 4). Note that although abundant CD4+ and β7+ cells are detected in the intestine of normal macaques (Figure 3a) most of both phenotypes are selectively eliminated within days of infection (Figure 3b). Finally, in animals infected with SIV or SHIV that were controlling plasma viremia, β7HIGHCD4+ T cells in the intestine were only partially restored (Figure 3b). Notably, β7HIGHCD8+ T cells were not depleted in the intestine or any other tissues at any stage of SIV infection (Figure 3 and data not shown). Furthermore, immunohistochemistry of intestinal tissues from animals in early SIV infection suggested a dramatic loss of α4β7+ CD4+ T cells (but not CD8+ T cells) consistent with selective lysis of these cells in the lamina propria (Figure 4).
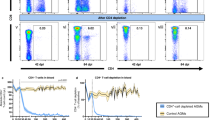
Changes in intestinal CD4+CD3+ T cells and β7HIGHCD4+ T cells throughout SIV infection. (a) Changes in intestinal CD4+CD3+ T cells (black line) and β7HIGHCD4+ T cells (dotted line) in SIV-infected macaques throughout infection. As expected, intestinal CD4+ T cells are markedly and persistently depleted by 13 days of inoculation, and this depletion persists throughout infection. β7HIGHCD4+ T cells are similarly persistently depleted in the intestine (dotted line), however, in animals controlling plasma viremia (controllers) there is a partial reconstitution of intestinal CD4+ T cells and as β7HIGHCD4+ T cells. (b) Dot plots from a representative macaque showing a marked loss of CD4+ T cells (top panels) and a profound and selective loss of β7HIGHCD4+ T cells (bottom panels) in the intestine. Error bars in a indicate s.e.m. SIV, simian immunodeficiency virus.
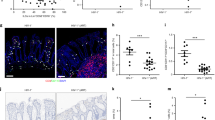
Visualization of β7+T cells in normal and SIV infected macaques. (a) Confocal microscopy of immunohistochemically stained jejunum sections showing β7 (green), CD4 (red), and CD3 (blue) expression. Note that β7 is normally expressed on T lymphocytes (CD3) in the intestinal mucosa. Also note that both β7+CD4+CD3+ (yellow-green/blue) T cells and β7+CD3neg cells (green–blue; presumably CD8+ T cells) are clearly evident in the intestine of normal macaques. (b) Jejunum section from an SIV-infected macaque 21 days after SIVmac251 inoculation. Note that both CD4+ (red) and CD4+β7+ (yellow-green/blue) T cells are now “missing” and yellow–green staining is limited to amorphous cellular debris, suggestive of marked, yet selective necrosis and lysis of CD3+CD4+β7+ T cells. Note that abundant CD3+CD4neg, β7+cells remain, indicating that non CD4+ T cells (CD8+) are spared. SIV, simian immunodeficiency virus.
As α4β7 directs homing of T cells specifically to intestinal tissues, and likely has a major role in the maintenance and/or reconstitution of intestinal CD4+ T cells, we hypothesized that a loss of circulating β7HIGHCD4+ T cells would correlate with the depletion and persistent loss of intestinal CD4+ T cells. As shown in Figure 5 detectable decrease in β7HIGHCD4+ T cells was observed in blood by 8 days of infection, and levels were significantly (P<0.01) lower than uninfected controls by 10 days of infection. Moreover, β7HIGHCD4+ lymphocytes continued to decrease in blood in parallel with intestinal CD4+ T cells throughout infection. In fact, levels of β7HIGHCD4+ T cells in blood closely reflected levels of intestinal CD4+ T cell changes throughout infection (Figure 5). Eventually, CD4+ T cells expressing β7HIGH in blood were essentially eliminated in macaques with AIDS, as were intestinal CD4+ T cells in these animals. Although there was a trend in chronic, asymptomatic infected animals to have higher levels of intestinal CD4+ T cells than macaques infected for 21 days (Figure 5), these differences were non-significant. However, in cohorts of animals controlling infection (with undetectable plasma viremia) β7HIGH CD4+ T cells were partially restored, which also directly correlated with levels of intestinal CD4+ T-cell restoration (Figure 5). Combined, this suggests that circulating β7HIGH cells may have a role in restoring intestinal CD4+ T cells, and tracking this T-cell subset in blood may be a useful surrogate to directly measuring intestinal CD4+ T cells in HIV-1 infection.
Loss of circulating β7HIGHCD4+ T cells in blood parallels the loss of intestinal CD4+ T cells throughout SIV infection. (a) Flow cytometry dot plots showing a rapid and persistent loss of β7HIGHCD4+ T cells in the blood. (b) Comparison of the loss of intestinal CD4+ T cells with the loss of β7HIGHCD4+ T cells in the peripheral blood throughout infection. Note that the loss of β7HIGHCD4+ T cells in blood directly parallels the loss of intestinal CD4+ T cells throughout SIV infection. Also note that the higher levels of intestinal CD4+ T cells in controllers is accompanied by higher levels of β7HIGHCD4+ T cells in the peripheral blood. Bars indicate s.e.m.
Comparison of the dynamics of β7HIGH and CCR5+ CD4+ T cells in blood during acute SIV infection
We and others previously hypothesized that the rapid and selective depletion of intestinal CD4+ T cells was due to a combination of their state of activation and expression of high levels of CCR5.8, 15, 16, 17, 19, 20, 21 Evidence for this included showing that activated CD4+CCR5+ memory T cells were eliminated at a higher rate than CCR5neg CD4+ T cells in the intestine.8, 15, 16 In addition, a selective loss of CD4+CCR5+ T cells in blood can be detected in individual macaques, at least in early SIV infection.8, 15, 16 However, as CCR5 is expressed on activated memory T cells in most tissues, and not restricted to intestinal T cells,22 we hypothesized that tracking β7HIGHCD4+ T cells in the blood could be a better surrogate marker for monitoring intestinal CD4+ T-cell depletion. We thus compared the dynamics of circulating β7HIGHCD4+ T cells with CCR5+CD4+ T cells in the blood in normal and SIV-infected animals with changes in intestinal CD4+ T cells. In normal macaques, percentages of circulating β7HIGHCD4+ T cells were much higher than CCR5+CD4+T cells (Figure 6a). Following SIV infection, both populations were rapidly and persistently depleted throughout infection, particularly in animals with AIDS. However, in animals controlling infection, blood CD4+CCR5+ T cells had returned to levels similar to uninfected controls (P>0.05), Thus, CD4+CCR5+ T cells in the blood were not reflective of the level of intestinal CD4+ T-cell depletion. In contrast, blood β7HIGHCD4+ T cells remained significantly lower in these “controllers” compared with uninfected animals (P<0.01) and the level of blood β7HIGHCD4+ T cells closely correlated with levels of CD4+ T cells remaining in the intestine. Finally, the rate and magnitude of β7HIGHCD4+ T-cell depletion in the blood was much higher than in CCR5+CD4+ T cells. As shown in Figure 6b, the slope of decline was −0.8 for circulating β7HIGHCD4+ T cells, vs. −0.3 for CCR5+CD4+ T cells, indicating a more rapid decline of the former in early infection.
Comparison of changes in β7HIGHCD4+ T cells and CCR5+CD4+ T cells from peripheral blood throughout SIV infection. (a) Comparison of peripheral blood β7HIGHCD4+, and CCR5+CD4+ T-cell subsets in normal and SIV-infected macaques. Note that there are significantly higher levels of β7HIGHCD4+ T cells than CCR5+CD4+ T cells in the blood of normal macaques. Although there is a detectable loss of CCR5+CD4+ T cells in the blood following SIV infection, it is neither as pronounced, nor as consistent in SIV-infected animals as the loss of β7HIGHCD4+ T cells. Further, in animals controlling infection, CCR5+CD4+ T cells are restored to levels similar to uninfected controls, whereas β7HIGHCD4+ T cells are only partially restored in the blood. (b) Comparison of the slope of decline of β7HIGHCD4+ T cells to CCR5+ T cells 13 days after SIV infection. Note that there is a more rapid and selective depletion of β7HIGHCD4+ T cells in the blood as compared with CCR5+CD4+ T cells in SIV infection. SIV, simian immunodeficiency virus.
We also compared the dynamics of both circulating β7HIGHCD4+ T and CCR5+CD4+ T cells to changes in intestinal CD4+ T cells by linear regression analysis (Figure 7). Although both populations showed significant correlations with the intestinal CD4+T cell loss, the r2 value of the β7HIGHCD4+ T cells was 0.6, whereas for CCR5+CD4+T cells it was 0.3 (Figure 7). Combined, this suggests that circulating β7HIGHCD4+ T cells may be a better surrogate marker for monitoring intestinal CD4+ T-cell depletion than circulating CCR5+CD4+ T cells.
Linear regression analysis comparing the percentage of intestinal CD4+ T cells peripheral blood β7HIGHCD4+ T cells (a), and CCR5+CD4+ T cells (b) in normal and SIV-infected macaques. Although a positive correlation is observed in both, note there is a much stronger positive correlation between circulating β7HIGHCD4+ T cells and intestinal CD4+ T cells than for circulating CCR5+CD4+ T cells. Correlation coefficients were determined using the Spearman's coefficient of correlation and linear regression was used to obtain r2 values. SIV, simian immunodeficiency virus.
Discussion
It is well established that SIV and HIV-1 primarily target intestinal CD4+ T cells, particularly in early infection.2, 17, 20, 23, 24 However, the intestinal mucosal immune system consists of distinct immune inductive and effector compartments, both harboring large numbers of CD4+ T cells, but with different phenotypic and functional characteristics. Understanding the nature of these distinct sites and cells is particularly important for understanding the pathogenesis of HIV-1 infection.
Large numbers of “resting, naive” CD4+ T cells reside in intestinal immune inductive sites (Peyer's patches and organized lymphoid follicles) also known as GALT, which are distributed primarily in the terminal small intestine (ileum) and large intestine. When resting T cells in GALT become activated through exposures to antigens they rapidly leave the GALT, migrate through the mesenteric lymph nodes, briefly recirculate in the blood, and eventually “home” to the diffuse intestinal lamina propria, which is distributed throughout the intestinal tract. This homing and dispersal of activated T cells throughout the intestine is mediated by a variety of chemokines and adhesion molecules, among which the interaction of the integrin α4β7 on leukocytes and mucosal addressin cell adhesion molecule-1 on postcapillary venules is particularly important.7, 25, 26 This mechanism allows for antigen-specific immune responses elicited in a single site to be rapidly disseminated throughout the intestinal tract. Thus, α4β7+ CD4+ T cells detected in the blood are believed to be recently activated, transitional or effector memory CD4+ T cells, which are selectively homing to intestinal effector tissues.27 The data presented here indicate that a loss of this particular subset in blood correlates with the massive CD4+ T-cell depletion that occurs in the intestinal lamina propria in SIV, and presumably HIV-1 infection. This loss could either be the result of selective infection and destruction of these cells, or alternatively, may reflect the failure of the central memory pool of CD4+ T cells to generate sufficient numbers of effector memory CD4+ T cells that can repopulate the intestine. Failure of this central memory pool has recently been hypothesized to have a major role in the development of immunosuppression and AIDS.28
Within days of infection, large numbers of memory CD4+ T cells are depleted in the intestinal lamina propria of HIV-infected humans, SIV-infected macaques, as well as non-progressing hosts such as sooty mangabeys and African green monkeys.2, 17, 20, 23, 24 We and others have proposed that this rapid and selective depletion of intestinal memory CD4+ T cells is mediated through direct viral infection and killing of these cells because of their high expression of CCR5 (permitting cells to be infected) and their state of activation, as HIV-1 selectively replicates in activated CD4+ T cells.8, 15, 16, 18 In support of this, selective loss of activated memory CCR5+CD4+ T cells can be detected in the intestine and other tissues in early SIV infection.8, 15, 16 However, memory CCR5+CD4+ T cells are not limited to mucosal tissues. In early SIV infection, a selective loss of CCR5+CD4+ T cells can clearly be detected in the peripheral blood. As SIV induces generalized immune activation, and because CCR5+ T cells are relatively rare in the peripheral blood, chronically infected animals may occasionally show similar or even higher levels of CD4+CCR5+ T cells in blood when compared with uninfected controls (unpublished observations). Furthermore, higher levels of CD4+CCR5+ T cells have been reported in the blood of HIV-1 infected humans.29 Finally, in our cohort of animals controlling infection, CD4+CCR5+ T cells seemingly returned to normal levels, similar to uninfected controls, despite persistently low levels of intestinal CD4+ T cells, indicating that CCR5 expression in blood does not reflect the magnitude of intestinal CD4+ reconstitution. Thus, CCR5 levels in blood have not proven to be a reliable marker of systemic CD4+ T-cell depletion in chronic SIV or HIV-1 infection. Possible reasons for this include the lower frequency of CCR5+ T cells in the circulation compared with α4β7 CD4+ T cells, as well as the possibility CCR5 is dynamically upregulated in response to non-specific inflammation in HIV infection, masking the effects of a selective depletion of these cells, at least in the blood.
Recently, Arthos et al.5 found that the HIV-1 envelope protein, gp120, binds to and signals by means of an activated form of integrin α4β7 on CD4+ T lymphocytes. These data suggest that α4β7 expression on CD4+ T cells may have a more direct role in depletion of CD4 cells, and in the infection and persistence of SIV/HIV in intestinal tissues than previously recognized. The affinity of gp120 for α4β7 could have several roles in the selective depletion of CD4+ T cells in intestinal or other tissues including; (i) using the α4β7 integrin for attachment and selective transport of the virus to intestinal tissues; (ii) facilitating direct infection of CD4+CCR5+ T cells coexpressing α4β7 by approximating the virus to its major coreceptors; (iii) activating α4β7HI cells potentially resulting in increased CCR5 expression, facilitating direct infection, and finally; (iv) facilitating infection of other cells by activation and formation of viral synapses, facilitating cell-to-cell spread of HIV-1.5
These data show that the selectivity and degree of β7HIGHCD4+ T-cell depletion in the blood during SIV infection parallels the loss of intestinal lamina propria CD4+ T cells. Although the mechanisms behind this are not known, this may be due to increased activation (and thus more viral replication) of these cells because of selective binding of gp120–α4β7 on these cells. In vitro, gp120/α4β7 binding has been shown to increase expression of LFA-1, consistent with cell activation.5 Conceivably, such activation may also increase the expression of CCR5 or other HIV-1 receptors, allowing cells to be more permissive to infection, or to support higher levels of viral replication. Although α4β7+ is not considered to be a receptor for viral entry into cells the loss of CD4+α4β7+ T cells appears to be the result of killing of these cells, as there is clearly death and lysis of CD4+α4β7+ T cells (but not CD3+CD4neg cells) in the intestine in early SIV infection (Figure 4).
Finally, these data suggest a persistent and dramatic loss of β7HIGHCD4+ T cells in blood may correlate with progression to AIDS, and that restoration of β7HIGHCD4+ T cells may reflect the levels of partial reconstitution of intestinal CD4+ T cells. Thus, α4β7 may be critically involved in the pathogenesis of the intestinal CD4+ T-cell loss, and the development of AIDS in general, at least in SIV-infected macaques. Although responses in HIV-infected humans may differ, others have indeed shown a marked and persistent loss of α4β7+ CD4+ T cells in HIV-infected patients, even when on anti-retroviral therapy.30 Although these investigators did not examine intestinal CD4+ T cells, these studies combined with others showing persistent loss of intestinal CD4+ T cells at all stages of HIV infection suggest that tracking β7 expression in blood may be a useful surrogate marker for monitoring intestinal CD4+ T-cell depletion and reconstitution in HIV-1 vaccine and therapeutic studies. Clearly, more studies are needed to validate these findings in HIV-infected patients, but these results suggest that α4β7 expression may have a major role in the pathogenesis of HIV infection and the development of AIDS.
Materials and Methods
Animals and virus
A total of 46 adult rhesus macaques (Macaca mulatta) of Indian origin on SIV pathogenesis studies were examined in this study. All animals were housed at the Tulane National Primate Research Center in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Of these, five uninfected animals were killed for tissue collection as controls, and another 41 were infected with either SIV or SHIV as follows; Thirty-seven macaques were infected with SIV either intravenously (n=27), intrarectally (n=6) or intravaginally (n=4) with either wild type SIVmac251 (n=31), SIVdeltaB670 (n=2)(viruses provided by Preston Marx) or molecularly cloned SIVmac239 (n=4) (virus courtesy of Ronald Desrosiers). These 37 SIV-infected macaques were humanely killed for tissue collection at the time points shown in Figure 3, including early (8 days (n=4), 10 days (n=3), 13 days (n=3) and 21 days (n=4)) post infection, chronic infection with either no overt signs of disease (chronic asymptomatic, n=8) or with illness that could not be definitively attributed to AIDS (e.g., nonresponsive diarrhea, weight loss, etc; n=6), and nine animals with overt signs of AIDS. All animals killed early in infection (21 days or less) were intravenously infected with SIVmac251 to reduce variation that can occur with mucosal inoculations, but we grouped chronically infected animals irrespective of inoculum or route because we have found that the pathogenesis of chronic infection is similar with these viruses, regardless of the route of inoculation. SIV-infected macaques killed with AIDS (n=9), all had AIDS-defining lesions and/or opportunistic infections including Pneumocystis carinii pneumonia (n=6); disseminated Mycobacterium avium infection (n=2) or SIV encephalitis (n=1). The remaining four animals (“controllers”) refers to four macaques infected with either SIVmac251 (n=1), SHIV89.6P (n=2) or SHIV-KU (n=1) which became infected and originally had high-peak plasma viremia, but subsequently controlled viral replication to undetectable levels (<125 copies per ml) in plasma. Intestinal samples from these animals were collected by endoscopic biopsy, as they remain clinically healthy.
Cell isolation and flow cytometry
Tissues were collected from the jejunum, ileum, colon, spleen, mesenteric, and axillary lymph nodes, and thymus within minutes of necropsy and transported to the lab on ice for immediate processing. Lymphocytes from the intestine and other tissues were isolated and stained for flow cytometry as previously described.15 Peripheral blood, spleen, and bone marrow cells were stained using a whole blood lysis technique as previously described.15 Blood and intestinal lymphocytes from all 46 animals were examined by four color flow cytometry with fluorescently conjugated monoclonal antibodies to CD4-APC, CD8-PerCP, CD45RA-FITC, or CD3-FITC combined with CCR5-PE (clone 3A9), or β7 integrin-PE (BD Biosciences, San Diego, CA) in separate tubes. Samples were acquired on a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed with Flowjo software (Tree star, Ashland, OR). To further characterize β7HIGHCD4+ cells in blood, an additional five normal macaques were examined by nine-color flow cytometry using appropriately diluted, directly conjugated monoclonal antibodies to CD95-FITC or CD45RA-FITC, β7 integrin-PE, CD49d/integrin α4-PE-Cy5, HLADR-PE-Cy7, CD28-APC, CD69-APC-Cy7, CD3-Pac. Blue (BD Biosciences) CD8- PE-TR (Caltag Laboratroies, Carlsbad, CA), and CD4-Qdot655 (NIH), followed by red blood cell lysis and wash with dPBS/BSA. These samples were resuspended with BD Stabilizing Fixative (BD Biosciences) and acquired on LSRII flow cytometer (Becton Dickinson). Data were analyzed with Flowjo software (Tree star).
Immunohistochemistry
Three color immunofluorescent staining for β7, CD4, and CD3 (T cells) was performed on selected animals (uninfected and through day 21 infection) to visualize and phenotype the distribution of β7+ T-cell subsets in tissues by confocal microscopy as previously described.31 In brief, tissues were stained using unconjugated primary antibodies and then with secondary antibodies conjugated to either Alexa 488 (green), Alexa 568 (red) or Alexa 633 (blue)(Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices represent 0.2 mm, and 32–62 optical slices were collected at 512× 512 Q1 pixel resolution. NIH Image (version 1.62) and Adobe Photoshop (version 7.0) were used to assign colors to the channels collected.
Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism 4.0 (GraphPad Software, SanDiego, CA). Comparisons between three or more groups were analyzed by a one-way ANOVA and Dunnett's Multiple Comparison Test for significant differences (P<0.05). The Dunnett's test was also used when comparing controls (one group) to other groups. Correlations between samples were calculated and expressed using the Spearman's coefficient of correlation. The rate of cell loss was determined using Linear Regression analysis.
References
Mehandru, S. et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med 3, e484 (2006).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 77, 11708–11717 (2003).
Guadalupe, M. et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol 80, 8236–8247 (2006).
Veazey, R.S. & Lackner, A.A. Impact of antiretroviral therapy on intestinal lymphoid tissues in HIV infection. PLoS Med 3, e515 (2006).
Arthos, J. et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol 9, 301–309 (2008).
Berlin, C. et al. a4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422 (1995).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Veazey, R.S. et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol 74, 11001–11007 (2000).
Poles, M.A., Elliott, J., Taing, P., Anton, P.A. & Chen, I.S. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol 75, 8390–8399 (2001).
Lackner, A.A. & Veazey, R.S. Current Concepts in AIDS Pathogenesis: insights from the SIV/Macaque Model. Annu Rev Med 58, 461–476 (2007).
Andrew, D.P., Rott, L.S., Kilshaw, P.J. & Butcher, E.C. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol 26, 897–905 (1996).
Rodriguez, M.W., Paquet, A.C., Yang, Y.H. & Erle, D.J. Differential gene expression by integrin beta 7+ and beta 7- memory T helper cells. BMC Immunol 5, 13 (2004).
Ericsson, A., Svensson, M., Arya, A. & Agace, W.W. CCL25/CCR9 promotes the induction and function of CD103 on intestinal intraepithelial lymphocytes. Eur J Immunol 34, 2720–2729 (2004).
Mora, J.R. & von Andrian, U.H. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol 27, 235–243 (2006).
Veazey, R.S. et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J. Virol 74, 57–64 (2000).
Mattapallil, J.J., Douek, D.C., Hill, B., Nishimura, Y., Martin, M. & Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005).
Brenchley, J.M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 200, 749–759 (2004).
Schnittman, S.M., Lane, H.C., Greenhouse, J., Justement, J.S., Baseler, M. & Fauci, A.S. Preferential infection of CD4+ memory cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci 87, 6058–6062 (1990).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–1371 (2006).
Veazey, R.S., Marx, P.A. & Lackner, A.A. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol 22, 626–633 (2001).
Picker, L.J. et al. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol 18, 399–405 (2006).
Mackay, C.R. & Sallusto, F. A new role for CCR5 in innate immunity--binding to bacterial heat shock protein 70. Eur J Immunol 36, 2293–2295 (2006).
Veazey, R.S. et al. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200, 761–770 (2004).
Berlin, C. et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Briskin, M. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 151, 97–110 (1997).
Campbell, D.J. & Butcher, E.C. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med 195, 135–141 (2002).
Okoye, A. et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med 204, 2171–2185 (2007).
Ostrowski, M.A. et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol 161, 3195–3201 (1998).
Krzysiek, R. & AOPAS Group Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood 98, 3169–3171 (2001).
Borda, J.T. et al. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am J Pathol 165, 2111–2122 (2004).
Acknowledgements
We thank Linda Green, Janell LeBlanc, Maryjane Dodd, Kelsi Rasmussen, Robin Rodriguez, and Maury Duplantis for their technical assistance, and Julie Bruhn, Calvin Lanclos, and Desiree Waguespacek for their expertise in flow cytometry. We thank Jason Dufour and Morgan Singletary for their veterinary expertise and the animal care staff for animal care. The work was supported in part by NIH Grants RR00164, AI049080, AA013563, and AI062410. The authors have no conflicting or competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Xu, H., Gill, A. et al. Monitoring α4β7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol 2, 518–526 (2009). https://doi.org/10.1038/mi.2009.104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2009.104
This article is cited by
-
MAdCAM costimulation through Integrin-α4β7 promotes HIV replication
Mucosal Immunology (2018)
-
Preferential loss of gut-homing α4β7 CD4+ T cells and their circulating functional subsets in acute HIV-1 infection
Cellular & Molecular Immunology (2016)
-
Impaired Th17 polarization of phenotypically naive CD4+ T-cells during chronic HIV-1 infection and potential restoration with early ART
Retrovirology (2015)
-
Binding of HIV-1 virions to α4β7 expressing cells and impact of antagonizing α4β7 on HIV-1 infection of primary CD4+ T cells
Virologica Sinica (2014)
-
Conditionally-live attenuated SIV upregulates global T effector memory cell frequency under replication permissive conditions
Retrovirology (2013)