Abstract
Conventional photoluminescence (PL) yields at most one emitted photon for each absorption event. Downconversion (or quantum cutting) materials can yield more than one photon by virtue of energy transfer processes between luminescent centers. In this work, we introduce Gd2O2S:Tm3+ as a multi-photon quantum cutter. It can convert near-infrared, visible, or ultraviolet photons into two, three, or four infrared photons of ∼1800 nm, respectively. The cross-relaxation steps between Tm3+ ions that lead to quantum cutting are identified from (time-resolved) PL as a function of the Tm3+ concentration in the crystal. A model is presented that reproduces the way in which the Tm3+ concentration affects both the relative intensities of the various emission lines and the excited state dynamics and providing insight in the quantum cutting efficiency. Finally, we discuss the potential application of Gd2O2S:Tm3+ for spectral conversion to improve the efficiency of next-generation photovoltaics.
Similar content being viewed by others
Introduction
Over the last decade, advanced luminescent materials exhibiting downconversion, also known as quantum cutting or quantum splitting, have been developed1,2. In this process, a high-energy photon is converted into two or more lower-energy photons, with a quantum efficiency of potentially well over 100%3. If the downconverted photons are in the visible range, this concept is of interest for color conversion layers in conventional lighting applications1,2,4,5,6. New exciting possibilities of downconversion to infrared (IR) photons lie in next-generation photovoltaics, aiming at minimizing the spectral mismatch losses in solar cells7,8,9,10,11.
Spectral mismatch is the result of the broad width of the spectrum emitted by the sun. Semiconductor absorber materials absorb only photons with an energy hν higher than the band gap Eg7. To absorb many photons and produce a large electrical current, absorber materials must therefore have a small bandgap. However, because excited charge carriers rapidly thermalize to the edges of a semiconductor’s conduction and valence bands, a high voltage output requires an absorber with a large band gap. Hence, materials with low transmission losses (leading to a large current) have high thermalization losses (leading to a low voltage) and vice versa12,13. These spectral mismatch losses constitute the major factor defining the relatively low Shockley–Queisser limit14, the maximum light-to-electricity conversion efficiency of 33% in a single-junction solar cell, obtained for a band gap of approximately 1.1 eV (1100 nm)13.
Efficiencies higher than 33% can be reached with multi-junction solar cells, where a stack of multiple absorber materials are each optimized to efficiently convert different parts of the solar spectrum to electricity15,16. Although efficiencies of greater than 40% have been obtained with this concept16, the devices are expensive and have limited tunability because of the requirement of high-quality contacts between absorber materials with matching physical and chemical properties.
An alternative solution to beat the Shockley–Queisser limit is by optical conversion of the solar spectrum prior to entering the solar cell2,7,8,9,10,11. A large number of downconversion materials able to convert high-energy photons into multiple lower-energy photons have recently been developed17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. The downconversion process occurs by energy transfer between luminescent centers, either lanthanide ions with a wide range of energy levels or organic molecules with low-energy triplet states31,32. If the IR photons generated are all absorbed by the solar cell material, the result can be a huge increase in the current output10,11,12,13,33. This concept does not impose requirements on the contact surface between the conversion layer and the solar cell, so it can relatively easily be integrated into existing solar cell technologies.
In this work, we introduce the quadruple-downconversion material Gd2O2S:Tm3+. Depending on the excitation energy, it can yield two, three, or even four IR photons (mainly approximately 1800 nm) per absorption event. The high-energy photons are cut by a series of cross-relaxation steps, where the initially excited Tm3+ ion transfers part of its energy to a neighboring Tm3+. By examining the emission spectra and dynamics as a function of Tm3+ concentration in the Gd2O2S host crystal, we determine the operative cross-relaxation processes leading to four-photon quantum cutting and quantify the rates and efficiencies. We find that at a moderate Tm3+ concentration of 10%, the excitation energy is very efficiently cut into IR quanta (in the 3H5 level emitting at 1215 nm and in the 3F4 level emitting at 1800 nm), with efficiencies of 199% for near-IR excitation at 800 nm, 298% for blue excitation at 470 nm, and 388% for ultraviolet (UV) excitation at 365 nm. We discuss the practical application of this multiple-downconversion material to increase the spectral response of solar cells (in particular Ge, of which the band gap is matched to emission from the 3F4 level). Efforts should be aimed at reducing losses due to concentration quenching of the 3F4 level and increasing the absorption by means of a sensitizer. In addition to applications in next-generation photovoltaics, because the excitation can be in the first ‘tissue-transparent spectral window’ of approximately 800 nm and the emission of 1800 nm lies in a spectral window with low tissue absorption and reduced light scattering34,35, the concept of efficient downconversion in Tm3+ may be useful for bio-imaging applications.
Materials and methods
Gd2O2S:Tm3+ powder samples
Microcrystalline samples of Gd2O2S:Tm3+ (x%) with x = 0.1, 1.0, 5.0, and 10.0 were prepared for this research by Tailorlux GmbH (Münster, Germany). The crystal structure was confirmed using X-ray powder diffractometry (see Supplementary Fig. S1). Gd2O2S has a  space group, where Tm3+ dopant ions substitute for Gd3+ at a site with point symmetry C3v surrounded by four oxygen and three sulfur atoms36,37.
space group, where Tm3+ dopant ions substitute for Gd3+ at a site with point symmetry C3v surrounded by four oxygen and three sulfur atoms36,37.
Experimental details
Emission spectra and photoluminescence (PL) decay curves were recorded using an Edinburgh Instruments FLS920 spectrofluorometer equipped with a 450 W xenon lamp. Visible emission was detected by a Hamamatsu R928 photomultiplier tube (400–850 nm). Emission spectra in the IR were recorded on a liquid-nitrogen cooled Hamamatsu R5509-72 photomultiplier tube (1100–1600 nm) and a thermoelectrically cooled G5852 InGaAs PIN photodiode (1500–2100 nm). For PL emission measurements, the samples were excited with the xenon lamp. For PL decay measurements, an optical parametric oscillator (OPO) system (Opotek HE 355 II) pumped by the third harmonic of a Nd:YAG laser (pulse width 10 ns; repetition rate 20 Hz) was used for excitation in the 3F2,3 (Figure 1c) or 1G4 (Figure 2c) levels. PL decay of the 1D2 level (Figure 3c) was excited with an Ekspla NR342B-10-SH/DUV OPO system (pulse width 5 ns; repetition rate 10 Hz). All experiments were performed at room temperature.
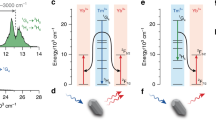
One-to-two downconversion from the 3F2,3 and 3H4 levels. (a) Emission spectra of Gd2O2S:Tm3+ (x%) with x = 0.1 (black), 1 (red), 5 (yellow), and 10 (blue) excited in the 3F2,3 level at 697 nm. The spectra are recorded on three different detectors, as indicated by the vertical dashed lines, and are normalized for each detector individually. (b) Possible decay pathways of the Tm3+ 3F2,3 excited state. Pathways I and II dominate at low Tm3+ concentrations; III and IV dominate at high concentrations. Multiphoton relaxation is depicted as dashed downward arrows, photon emission as colored arrows with symbols matching those marking emission lines in a, and cross-relaxation as black solid arrows. (c) Decay dynamics of the 3H4 → 3H6 emission at 800 nm upon excitation in the 3F2,3 level at 697 nm. Solid lines are fit to Equation (2), from which we obtain an intrinsic lifetime of τ0 = 255 µs and a critical radius for cross-relaxation of R0 = 9.3 Å. (d) The ratio between the 3F4 and 3H4 emission intensities as obtained from the spectra shown in a (circles) normalized to the ratio at 0.1% Tm3+. The solid line is the ratio expected based on the dynamics of c. (e) The theoretical absolute photon yields from the 3H4 level (green), 3F4 level (red), and the total yield (black) following excitation into the 3H4 (or 3F2,3) level.
One-to-three downconversion from the 1G4 level. (a) Emission spectra of Gd2O2S:Tm3+ (x%) with x = 0.1 (black), 1 (red), 5 (yellow), and 10 (blue) excited in the 1G4 level at 470 nm. They are recorded on three different detectors, as indicated by the vertical dashed lines, and are normalized for each detector individually. (b) Possible decay pathways of the Tm3+ 1G4 excited state include cascade processes (pathways I, II, and III). At higher Tm3+ concentrations, (1G4, 3H6) → (3F2,3, 3F4) cross-relaxation becomes the dominant decay pathway (IV and V), followed by further cross-relaxation from the 3F3,2 and 3H4 levels (see Figure 1). (c) The decay dynamics of the 1G4 level in Gd2O2S:Tm3+ (x%) with x = 0.1 (black), 1 (red), 5 (yellow), and 10 (blue), excited at 465 nm and detected for the 1G4 → 3F4 emission at 653 nm. Solid lines are fits to Equation (2), yielding an intrinsic lifetime of τ0 = 105 μs and a critical radius for cross-relaxation of R0 = 10.4 Å. (d) The intensity of emission from the 3F4 (red circles) and 3H4 (solid green triangle for 3H4 → 3F4, open green triangles for 3H4 → 3H6) levels relative to the 1G4 level. Solid lines are the ratios as expected from our model (see Supplementary Information). The dashed green line would be the expected ratio of 3H4 emission to 1G4 emission if only the first step in the sequence of cross-relaxations occurred, i.e., (1G4, 3H6) → (3F2,3, 3F4). The agreement with the experiment is very poor (compare to the green triangles), proving the occurrence of multiple cross-relaxation steps. (e) The total downconversion efficiency from the 1G4 level (black line) and separated by emission from the 1G4 (blue), 3H4 (green), and 3F4 (red) levels.
One-to-four downconversion from the 1D2 level. (a) Emission spectra of Gd2O2S:Tm3+ (x%) with x = 0.1 (black), 1 (red), 5 (yellow), and 10 (blue) excited in the 1D2 level at 365 nm. (b) Decay pathways from the 1D2 level. Pathways I, II, and III involve cascade emission of photons (colored downward arrows marked with symbols corresponding to those in a) and are operative at low Tm3+ concentration. At high Tm3+ concentrations, cross-relaxation (solid black arrows in pathways IV and V) becomes more efficient, effectively cutting the excitation energy in four and leading to emission in the IR (yellow square and red circle in a). (c) PL decay curves of the 1D2 level recorded at 465 nm (1D2 → 3F4). Solid lines are fits to Equation (2), yielding an intrinsic lifetime of τ0 = 4.0 µs for the 1D2 level and a critical radius for cross-relaxation of R0 = 7.1 Å. (d) The emission intensity from 3F4 (red circles) and 3H4 (green filled triangles: 3H4 → 3F4; green open triangles: 3H4 → 3H6) relative to 1D2. Solid lines are the trends expected based on our model (see Supplementary Information). If we include only the first cross-relaxation step in our model (i.e., (1D2, 3H6) → (3H4, 3F2,3)), the predicted intensity ratio between 3H4 and 1D2 (dashed green line) deviates strongly from the experimental data points. (e) The total downconversion quantum yield (black line) following 1D2 excitation. Emission comes mainly from the 1D2 level at low Tm3+ concentrations and from 3F4 at high Tm3+ concentrations. Consistent with the experiment (a), emission from the 3H4 level is brightest at intermediate concentrations of approximately 1%.
Modeling the photon yield from downconversion
To model the efficiencies of the downconversion processes in Gd2O2S:Tm3+, we use the overview of possible relaxation pathways (see Figures 1b, 2b, and 3b). For the cascade emissions, which dominate the downconversion efficiency at low Tm3+ concentration, we use the branching ratios as determined by Yi et al.38 for a crystal structure (Y2O3) similar to ours (Gd2O2S). For the cross-relaxation processes, which lead to efficient downconversion at high Tm3+ concentrations (>1%), we fit the excited state decay dynamics (see Figures 1c, 2c, and 3c) of the 3H4, 1G4, and 1D2 levels to a model of first-order energy transfer17,22,39,40 (Equations (1) and (2)) and obtain values for the cross-relaxation strengths Cxr. From these, the cross-relaxation efficiency ηxr is calculated. For more details, see the Supplementary Information.
Results and discussion
One-to-two downconversion following excitation in the near-infrared
We start by examining the downconversion from the 3H4 and 3F2,3 levels of Tm3+. Figure 1a shows the emission spectra of Gd2O2S:Tm3+ (x%) upon excitation in the 3F2,3 level at 697 nm, from bottom to top for increasing Tm3+ concentration of x = 0.1 (black), 1 (red), 5 (yellow), and 10 (blue). The spectra are recorded on three separate detectors (boundaries indicated with vertical dashed lines) and are normalized for each detector separately. Consequently, the relative intensities of emission lines can be compared between different Tm3+ concentrations but not between different detectors. The emission lines are ascribed to the 3H4 → 3H6 transition at 800 nm, the 3H5 → 3H6 transition at 1215 nm, the 3H4 → 3F4 transition at 1450 nm, and the 3F4 → 3H6 transition at 1800 nm21,29,41. At low Tm3+ concentrations (0.1%, 1%), the emission is mainly from the 3H4 level (lines indicated with a green triangle), whereas at higher concentrations (5%, 10%), emissions from 3H5 (yellow square) and 3F4 (red circle) dominate.
The observations are consistent with the relaxation pathways illustrated in Figure 1b. Pathways I and II dominate at low Tm3+ concentrations. Pathways III and IV, which involve cross-relaxation between pairs of ions and result in downconversion, become more likely at higher Tm3+ concentrations. Colored downward arrows represent photon emission, with symbols matching those marking the emission lines in Figure 1a. Fast non-radiative 3F2,3 → 3H4 multi-phonon relaxation (dashed downward arrow) is evidenced by the absence of emission from the 3F2,3 level. The occurrence of cross-relaxation processes (black solid arrows in pathways III and IV) is evidenced by the supralinear increase of emission intensity from the 3H5 and 3F4 levels with increasing Tm3+ concentration. Whereas radiative decay from the 3F2,3 level cannot compete with multi-phonon relaxation, cross-relaxation at high Tm3+ concentrations (pathway IV) can, as evidenced by the observation of 3H5 emission at 1215 nm. This indicates that very fast rates are possible for cross-relaxation processes in Gd2O2S:Tm3+. Indeed, Supplementary Fig. S2 shows that no 3H5 emission is observed under excitation directly in the 3H4 level (rather than in the 3F2,3 level) because pathway IV is not possible. This also proves that multi-phonon relaxation from 3H4 to 3H5 is negligible.
To quantify the rates and efficiencies of downconversion by cross-relaxation from the 3H4 state, we recorded PL decay curves excited in the 3F2,3 level (697 nm) and recorded at 800 nm at the 3H4 → 3H6 transition. Figure 1c shows that for increasing Tm3+ concentration, the decay of the 3H4 level rapidly becomes faster, consistent with cross-relaxation (pathway III in Figure 1b). We fit the decay curve for 0.1% Tm3+ to a single exponential, assuming no cross-relaxation, and obtain an intrinsic lifetime of the 3H4 level of τ0 = 255 µs. Next we assume that at higher Tm3+ concentrations, cross-relaxation can take place via dipole–dipole coupling in pairs of Tm3+ ions, yielding a cross-relaxation rate Γxr for a single pair proportional to the inverse sixth power of the separation r between the ions17,22,39,40:

The prefactor Cxr (which represents the strength of cross-relaxation) can alternatively be converted to a critical radius  , defined as the pair separation at which cross-relaxation has a 50% efficiency. We fit the decay curve for 1% Tm3+ to an analytical model that assumes a random substitution of Tm3+ for Gd3+ in the Gd2O2S crystal39,40:
, defined as the pair separation at which cross-relaxation has a 50% efficiency. We fit the decay curve for 1% Tm3+ to an analytical model that assumes a random substitution of Tm3+ for Gd3+ in the Gd2O2S crystal39,40:

where x is the Tm3+ acceptor concentration, and (ri, ni) represents the ‘neighbor list’ given by the crystal structure of Gd2O2S. The best fit is obtained for Cxr = 2.5 nm6 ms−1, corresponding to a critical radius of R0 = 9.3 Å. This translates into a cross-relaxation rate in a nearest-neighbor pair (at a separation of 3.5 Å; one Tm3+ in the 3H4 level and one in the 3H6 ground state) of 1/0.75 μs, 341× faster than radiative decay from the 3H4 level. In Figure 1c, the solid lines through the data for 5% (yellow) and 10% (blue) Tm3+ are drawn following Equation (2) without additional fit parameters. They show a good match with the experimental PL decay curves. Deviations at high Tm3+ concentrations may be due to energy migration over the Tm3+ sublattice, reabsorption of emitted light, or upconversion processes (i.e., the inverse of cross-relaxation; see Supplementary Information for further discussion).
Based on the possible decay pathways of the 3H4 level (Figure 1b) and with the assumption that no non-radiative loss occurs, we can estimate (see Supplementary Information for details) the expected light yield following excitation at 700–800 nm in the 3F2,3 or 3H4 level. Using the fitted value for the cross-relaxation strength Cxr (Figure 1c), we can estimate how the expected intensity ratio between 3F4 and 3H4 emission varies with Tm3+ concentration. In Figure 1d we plot the result (solid red line) and compare it to the experimental data (red circles). Efficient cross-relaxation causes the relative intensity in the IR at 1800 nm from the 3F4 level to increase by more than a factor 100 as the Tm3+ concentration increases from 0.1% to 10%. Hence, our model of (3H4, 3H6) → (3F4, 3F4) cross-relaxation (Figure 1b) via dipole–dipole interaction is consistent with both the excited state dynamics (Figure 1c) and the resulting emission intensities (Figure 1d). The deviation between experiment and model that occurs in Figure 1d at 10% Tm3+ can be understood in terms of concentration quenching of the 3F4 level (see the section on “Discussion”).
In Figure 1e, we plot the one-to-two downconversion quantum yield η2 (see Supplementary Information) following excitation into the 3H4 level as a function of Tm3+ concentration. We distinguish between photons emitted from the 3H4 level and those emitted from the 3F4 level based on our model and the fitted cross-relaxation strength Cxr. Whereas at low Tm3+ concentrations the emission comes primarily from the 3H4 level (green line), at higher concentrations, 3F4 emission (red line) is dominant. We see that Gd2O2S:Tm3+ can efficiently downconvert photons of 800 nm. Because of cascade emission (pathway II in Figure 1b), η2 is already over 100% (119%) at 0.1% Tm3+ 21,41,42. It increases to 199% at 10% Tm3+ because cross-relaxation becomes much more efficient. Experimentally, the absolute downconversion quantum yield is difficult to determine quantitatively because emissions occur over such a large spectral range, including very close to the excitation wavelength and beyond the detector range. We can see in Figure 1a that the absolute emission intensity from the 3F4 level decreases when the Tm3+ increases from 5% to 10%. This indicates that in our samples, concentration quenching effects (see the section on “Discussion”) are present at high Tm3+ concentrations, which diminish the actual quantum yields to below those estimated in Figure 1e.
One-to-three downconversion following blue excitation
Next, we investigate the efficiency of downconversion from the 1G4 level of Tm3+ 43. We perform a similar analysis as presented above in Figure 1 but for higher-energy excitation, to build a complete picture of the chain of cross-relaxation processes that can take place in Gd2O2S:Tm3+. The 1G4 level is excited with blue photons of 470 nm, which each provide enough energy to generate three IR photons of 1800 nm (3F4 → 3H6). Figure 2a shows emission spectra of Gd2O2S:Tm3+ following excitation in the 1G4 level at 470 nm. In addition to the emission lines observed in Figure 1a from 3H4 (green triangles), 3H5 (yellow square), and 3F4 (red circle), we now see emission from the 1G4 level (blue crosses). The line at 650 nm is from the 1G4 → 3F4 transition, whereas the emission at approximately 1200 nm, observed at low Tm3+ concentrations (0.1%, 1%), originates from the 1G4 → 3H4 transition. At high Tm3+ concentrations (5%, 10%), the spectrum at approximately 1215 nm is of a different origin, namely the 3H5 → 3H6 transition, as evident from a comparison to Figure 1a. Overall, we see that at the lowest Tm3+ concentration (0.1%), the emission comes mainly from the 1G4 level (blue crosses), at intermediate concentration (1%), 3H4 emission is relatively strong (green triangles), whereas at high concentrations (5%, 10%) the spectrum is dominated by 3H5 (yellow square) and 3F4 (red circle) emissions.
Based on these observations, we propose relaxation pathways for the 1G4 level as depicted in Figure 2b. At low Tm3+ concentrations, pathways I, II, and III result in cascade emissions from the 1G4 level (there can also be direct radiative decay to the ground state, not depicted). At high Tm3+ concentrations, (1G4, 3H6) → (3F2,3, 3F4) cross-relaxation occurs29,44, after which the 3F2,3 and 3H4 excitations are further downconverted as already discussed in Figure 1. The result at high Tm3+ concentrations (5%, 10%) can be three IR emissions for each blue excitation, consistent with the dominant contribution from the 3H5 (yellow square) and 3F4 (red circle) emissions in the spectra of Figure 2a. There may also be (1G4, 3H6) → (3H4, 3H5) and (1G4, 3H6) → (3H5, 3H4) cross-relaxation processes29,44, which would lead to similar downconversion schemes, except that the 3H5 level is directly populated in the first step of the sequence of cross-relaxation steps. Because we experimentally observe 3H5 emission only at the highest concentrations, the dominant first cross-relaxation process is probably (1G4, 3H6) → (3F2,3, 3F4).
We investigate the downconversion dynamics of the 1G4 level, as shown in Figure 2c. The solid lines are fits of the experimental data (symbols) to the model of cross-relaxation by dipole–dipole coupling (Equation (2)). We obtain for the 1G4 level an intrinsic lifetime of τ0 = 105 µs and a cross-relaxation strength of Cxr = 11.8 nm6 ms-1, corresponding to a critical radius of R0 = 10.4 Å. This means that the cross-relaxation rate of the 1G4 level in a nearest-neighbor pair is 1/0.16 µs, 660× faster than radiative decay. Such fast cross-relaxation rates explain why in upconversion experiments only low Tm3+ concentrations of no higher than 1% yield bright blue upconversion emission44,45,46.
As we did in Figure 1d for 3H4 excitation, with the fit results on the decay dynamics (Figure 2c), we can calculate the theoretical photon yields upon 1G4 excitation (see Supplementary Information). Figure 2d shows the intensity ratio of 3F4 (red) or 3H4 (green) emission to 1G4 emission. The experimental data (data points) show a good match to the theoretical predictions (solid lines). This confirms the occurrence of the sequence of cross-relaxation processes depicted in Figure 2b, which lead to the generation of three IR photons for each blue excitation. As a check, the dashed green line in Figure 2d shows the expected 3H4-to-1G4 intensity ratio in the hypothetical case that only the first cross-relaxation process occurred (i.e., (1G4, 3H6) → (3F2,3, 3F4)) rather than a sequence of two cross-relaxation steps. This scenario is not consistent with the experimental emission spectra.
In Figure 2e, we plot the absolute photon yields following 1G4 excitation, as calculated with our model (see Supplementary Information). At low Tm3+ concentrations, the emission comes mainly from the 1G4 level (blue line), although through cascade processes (pathways I, II, III in Figure 2b), the other levels emit as well. Consistent with the experimental spectra (Figure 2a), emission comes primarily from the 3F4 level (red line) at high Tm3+ concentrations. The total downconversion efficiency increases from 165% at 0.1% Tm3+ (by virtue of cascade emissions) to 298% at 10% (determined by cross-relaxation). In practice the absolute photon yield is limited by concentration quenching of the 3F4 emission at high Tm3+ concentrations.
One-to-four downconversion following UV excitation
Finally, we increase the excitation energy further to be resonant with the 1D2 level of Tm3+. Excitation photons in the UV (of 365 nm) have sufficient energy to yield as many as four IR photons from the 3F4 level. Figure 3a shows the emission spectra of Gd2O2S:Tm3+ excited in the 1D2 level at 365 nm. We observe emission from the 1D2 level (purple diamonds) and, at higher Tm3+ concentrations, from 3H4 (green triangles), 3H5 (yellow square), and 3F4 (red circle) but not from the 1G4 level (compare Figure 2a). This indicates that cross-relaxation processes occur but not to the 1G4 level. Moreover, we can conclude that multi-phonon relaxation from 1D2 to 1G4 is negligible.
In Figure 3b, we depict the decay pathways from the 1D2 level dominant in Gd2O2S:Tm3+. At low Tm3+ concentrations there are possibilities for cascade emission (pathways I, II, and III)29,46,47,48. At high Tm3+ concentrations, because we see in the emission spectrum that cross-relaxation to the 1G4 level is not efficient, the dominant process must be (1D2, 3H6) → (3H4, 3F2,3), as depicted in pathways IV and V29,44. After reaching the 3H4 and 3F2,3 levels, the Tm3+ ions decay further, by cross-relaxation, as discussed above in Figure 1. The result can be as many as four emitted IR photons (red circles and yellow squares) for each UV excitation.
Figure 3c shows the decay dynamics of the 1D2 level recorded for the 1D2 → 3F4 emission at 465 nm. We again apply our model of cross-relaxation by dipole–dipole interaction (Equation (2)) to fit the dynamics. The best fit is obtained for an intrinsic lifetime of τ0 = 4.0 μs and a cross-relaxation strength of Cxr = 32.8 nm6 ms−1 (corresponding to a critical radius of R0 = 7.1 Å, and a nearest-neighbor cross-relaxation rate of 1/0.06 μs, 69× faster than the intrinsic decay). Hence, this cross-relaxation process from the 1D2 level is less efficient than those from the 3H4 (Figure 1c) and 1G4 levels (Figure 2c) but still competes strongly with radiative decay.
To confirm the occurrence of one-to-four downconversion in Gd2O2S:Tm3+ via the scheme of three cross-relaxation steps (Figure 3b), we compare the relative emission intensities in the spectra to the predictions of our model (see Supplementary Information). Figure 3d shows a good correspondence between the experiment (data points) and the model (solid lines). Downconversion occurs at low Tm3+ concentrations because of several possible cascade processes (pathways I, II and III in Figure 3b). Nevertheless, the relative intensity of 3F4 emission increases by a factor 100 as the Tm3+ increases from 0.1% to 10%. As a check, we can see that if we do not include the second and third steps of the sequence of cross-relaxations, the agreement between model and experiment is very poor (dashed green line).
Figure 3e presents the theoretical photon yield for excitation in the 1D2 level. At the low Tm3+ concentration of 0.1%, the downconversion quantum yield is as high as 186% by virtue of the many possible cascade processes (pathways I, II, and III in Figure 3b), through which the UV excitation can relax42. The downconversion quantum yield increases to 388% at 10% Tm3+ because the cross-relaxation becomes more efficient. By comparing the absolute emission intensities (Figure 3a) from the 3F4 level for 5% and 10% Tm3+, it becomes apparent that concentration quenching effects are at play at the highest Tm3+ concentrations, which reduce the effective downconversion yield (see the section on “Discussion”).
Discussion
Our results highlight Gd2O2S:Tm3+ as a promising material to efficiently downconvert the near-IR, visible, and UV part of the spectrum to IR photons. Two challenges have to be solved for this material to significantly enhance the photo-response of next-generation solar cells: (i) the relatively weak absorption of Tm3+ and (ii) concentration quenching of the 3F4 level.
The absorption spectrum of Tm3+, as for most other trivalent lanthanide ions, contains a few narrow lines originating from the intraconfigurational transitions from the 3H6 ground state to the many possible excited states. In the inset of Figure 4a, we show the excitation spectra of Tm3+ for the 3H4 → 3F4 emission. The absorptions are relatively weak and narrow. A more detailed investigation of the different line intensities in the full excitation spectrum, as a function of Tm3+ concentration, is presented in Supplementary Fig. S3. To increase the effective absorption strength of Tm3+ and make it broadband, sensitizers are needed20,47,48,49,50. For example, the material could be co-doped with strongly absorbing centers, such as Ce3+, for sensitization20,39,49,50. Such schemes allow for the absorption spectrum of the material to be tuned independently of the downconversion process. This opens up interesting but challenging possibilities, such as materials that convert UV and near-IR to IR photons but are transparent to visible light for use as luminescent solar concentrators in windows.
Reducing spectral mismatch losses with Gd2O2S:Tm3+. (a) A simple solar cell with a band gap of 0.65 eV (1900 nm; for example, Ge) can effectively use only the part of the solar spectrum shaded red. Spectral conversion by a sensitized downconverter layer of Gd2O2S:Tm3+ would make additional parts available: the green part by one-to-two downconversion from the 3H4 level, the blue part by one-to-three downconversion from the 1G4 level, and the purple part by one-to-four downconversion from the 1D2 level. The inset shows the positions of the various absorption lines in Gd2O2S:Tm3+ in the excitation spectrum of the 3H4 → 3F4 emission at 1450 nm. (b) The calculated spectral losses as a function of the Tm3+ concentration in Gd2O2S and the quantum efficiency ηem of the IR emission from the 3F4 level. The solid black line shows how ηem varies with the Tm3+ concentration according to Equation (3), and assuming a critical concentration of x0 = 10%. For the actual crystal, where the concentration quenching is directly linked to the Tm3+ concentration, as described by Equation (3), the spectral losses as a function of the Tm3+ concentration are the color values at the position of the solid line. They are lowest (42%) at 2–3% Tm3+. The dashed line is the contour corresponding to the spectral losses of 50% for a crystalline Si solar cell (band gap = 1.1 eV). We see that a Gd2O2S:Tm3+ with a Ge solar cell can outperform crystalline Si.
Figure 4a shows the fraction of the solar spectrum made available through downconversion, if we assume broadband effective absorption of Tm3+ by means of sensitization. We consider a solar cell with a bandgap of 0.65 eV (1900 nm; e.g., Ge)11 such that all 3F4 emission can be absorbed. The red area in Figure 4a is directly usable by this solar cell. In the ideal sensitized downconverter (based on the excitation spectrum of Tm3+; Supplementary Fig. S3 and the inset of Figure 4a), all photons with energy higher than hν > 3.36 eV are funneled to the 1D2 level, photons of 2.59 eV < hν < 3.36 eV to the 1G4 level, and photons of 1.50 eV < hν < 2.59 eV to the 3H4 level. The colored areas in Figure 4a indicate the gain enabled by one-to-two (green), one-to-three (blue), and one-to-four (purple) downconversion. The spectral mismatch losses are reduced from 59% in the bare solar cell to 37% in combination with the downconverter (compared to 50% for a Si solar cell).
The essential last step of the downconversion is the emission of photons from the 3F4 level. As apparent from the relatively low absolute emission intensities at 10% Tm3+ (Figures 1a, 2a, and 3a), the 3F4 level is partially quenched at this concentration. This can be ascribed to concentration quenching, i.e., migration of the excitation energy over the Tm3+ sublattice until it reaches a quenching site. The effect has been studied in detail by Auzel et al.51 for the lowest excited state of Yb3+, Er3+, and Ho3+ in the crystalline host material Y2O3. They used a model where the PL quantum efficiency of emission depends on the concentration of luminescent ions x as

where x0 is the critical concentration. Auzel et al. found critical concentrations of approximately x0 ≈ 10% for the three ions investigated51. The effect of concentration quenching in a crystal doped with luminescent ions depends on the material quality52. With our equipment, we are unable to measure ηem for the IR emission from the lowest excited state of Tm3+ in our samples, but the values could be expected to follow the same trend as those found by Auzel et al. for Yb3+, Er3+, and Ho3+ in Y2O350.
In Figure 4b, we plot how spectral losses of the situation depicted Figure 4a (solar cell of band gap 0.65 eV plus Gd2O2S:Tm3+ downconverter) would depend on the Tm3+ concentration and ηem of the 3F4 level. The lowest spectral losses require a high Tm3+ concentration (for efficient cross-relaxation) and a high ηem. Because, as described by Equation (3), high Tm3+ concentrations come with a lower ηem, there is some optimum concentration. The solid black line in Figure 4b depicts how ηem would vary with Tm3+ concentration according to Equation (3) with x0 = 10%. The lowest spectral losses are 42% at a Tm3+ concentration of 2–3%, significantly lower than the 59% for the bare solar cell with a band gap of 0.65 eV and even lower than Si (50%; dashed line).
Conclusion
To summarize, we have demonstrated that Tm3+ in Gd2O2S can effectively downconvert high-energy photons from the near-IR, visible and UV to IR photons of a wavelength of 1800 nm. By virtue of a variety of cross-relaxation processes, an excitation in the 3H4 level (800 nm) can be converted to two IR photons, from the 1G4 level (470 nm) to three and from the 1D2 level (365 nm) to four. We have quantified the cross-relaxation rates and efficiencies for different Tm3+ concentrations by fitting the decay dynamics of the levels involved. For the three downconversion processes, the efficiencies are near 200%, 300%, and 400% for 10% Tm3+. These results are consistent with the relative intensities of the emission lines as found experimentally. We have discussed the potential application of Gd2O2S:Tm3+ as a spectral converter for photovoltaics. In particular, this material could greatly improve the efficiency of Ge solar cells, to such extent that they could even outperform crystalline Si in terms of spectral mismatch losses.
References
Wegh RT, Donker H, Oskam KD, Meijerink A . Visible quantum cutting in LiGdF4:Eu3+ through downconversion. Science 1999; 283: 663–666.
Zhang QY, Huang XY . Recent progress in quantum cutting phosphors. Prog Mater Sci 2010; 55: 353–427.
Dexter DL . Possibility of luminescent quantum yield greater than unity. Phys Rev 1957; 108: 630–633.
Jüstel T, Nikol H . Optimization of luminescent materials for plasma display panels. Adv Mater 2000; 12: 527–530.
Feldmann C, Jüstel T, Ronda CR, Schmidt PJ . Inorganic luminescent materials: 100 years of research and application. Adv Funct Mater 2003; 13: 511–516.
Lee TJ, Luo LY, Diau EWG, Chen TM, Cheng BM et al. Visible quantum cutting through downconversion in green-emitting K2GdF5:Tb3+ phosphors. Appl Phys Lett 2006; 89: 131121.
Trupke T, Green MA, Würfel P . Improving solar cell efficiencies by down-conversion of high-energy photons. J Appl Phys 2002; 92: 1668–1674.
Richards BS . Luminescent layers for enhanced silicon solar cell performance: down-conversion. Sol Energy Mater Sol Cells 2006; 90: 1189–1270.
van der Ende BM, Aarts L, Meijerink A . Lanthanide ions as spectral converters for solar cells. Phys Chem Chem Phys 2009; 11: 11081–11095.
Abrams ZR, Niv A, Zhang X . Solar energy enhancement using down-converting particles: a rigorous approach. J Appl Phys 2011; 109: 114905.
ten Kate OM, de Jong M, Hintzen HT, van der Kolk E . Efficiency enhancement calculations of state-of-the-art solar cells by luminescent layers with spectral shifting, quantum cutting, and quantum tripling function. J Appl Phys 2013; 114: 084502.
Luque A . Will we exceed 50% efficiency in photovoltaics? J Appl Phys 2011; 110: 031301.
Shockley W, Queisser HJ . Detailed balance limit of efficiency of p-n junction solar cells. J Appl Phys 1961; 32: 510–519.
Polman A, Atwater HA . Photonic design principles for ultrahigh-efficiency photovoltaics. Nat Mater 2012; 11: 174–177.
Martí A, Araújo GL . Limiting efficiencies for photovoltaic energy conversion in multigap systems. Sol Energy Mater Sol Cells 1996; 43: 203–222.
Green MA, Emery K, Hishikawa Y, Warta W . Solar cell efficiency tables (version 33). Prog Photovolt: Res Appl 2009; 17: 85–94.
Vergeer P, Vlugt TJH, Kox MHF, den Hertog MI, van der Eerden JPJM et al. Quantum cutting by cooperative energy transfer in YbxY1-xPO4:Tb3+. Phys Rev B 2005; 71: 014119.
Zhang QY, Yang GF, Jiang ZH . Cooperative downconversion in GdAl3(BO3)4:RE3+,Yb3+ (RE= Pr, Tb, and Tm). Appl Phys Lett 2007; 91: 051903.
Ye S, Zhu B, Luo J, Chen JX, Lakshminarayana G et al. Enhanced cooperative quantum cutting in Tm3+-Yb3+ codoped glass ceramic containing LaF3 nanocrystals. Opt Express 2008; 16: 8989–8994.
van der Ende BM, Aarts L, Meijerink A . Near-infrared quantum cutting for photovoltaics. Adv Mater 2009; 21: 3073–3077.
Yu DC, Zhang JP, Chen QJ, Zhang WJ, Yang ZM et al. Three-photon near-infrared quantum cutting in Tm3+-doped transparent oxyfluoride glass ceramics. Appl Phys Lett 2012; 101: 171108.
van Wijngaarden JT, Scheidelaar S, Vlugt TJH, Reid MF, Meijerink A . Energy transfer mechanism for downconversion in the (Pr3+,Yb3+) couple. Phys Rev B 2010; 81: 155112.
Zheng W, Zhu HM, Li RF, Tu DT, Liu YS et al. Visible-to-infrared quantum cutting by phonon-assisted energy transfer in YPO4:Tm3+, Yb3+ phosphors. Phys Chem Chem Phys 2012; 14: 6974–6980.
Eilers JJ, Biner D, van Wijngaarden JT, Krämer K, Güdel HU et al. Efficient visible to infrared quantum cutting through downconversion with the Er3+-Yb3+ couple in Cs3Y2Br9 . Appl Phys Lett 2010; 96: 151106.
Miritello M, Savio RL, Cardile P, Priolo F . Enhanced down conversion of photons emitted by photoexcited ErxY2-xSi2O7 films grown on silicon. Phys Rev B 2010; 81: 041411(R).
Yu DC, Huang XY, Ye S, Peng MY, Zhang QY et al. Three-photon near-infrared quantum splitting in β-NaYF4:Ho3+. Appl Phys Lett 2011; 99: 161904.
Chen DQ, Wang YS, Hong MC . Lanthanide nanomaterials with photon management characteristics for photovoltaic application. Nano Energy 2012; 1: 73–90.
Yu DC, Ye S, Huang XY, Zhang QY . Enhanced three-photon near-infrared quantum splitting in β-NaYF4:Ho3+ by codoping Yb3+. AIP Adv 2012; 2: 022124.
Chen XB, Salamo GJ, Yang GJ, Li YL, Ding XL et al. Multiphoton near-infrared quantum cutting luminescence phenomena of Tm3+ ion in (Y1-xTmx)3Al5O12 powder phosphor. Opt Express 2013; 21: A829–A840.
Timmerman D, Valenta J, Dohnalová K, de Boer WDAM, Gregorkiewicz T . Step-like enhancement of luminescence quantum yield of silicon nanocrystals. Nat Nanotechnol 2011; 6: 710–713.
Tabachnyk M, Ehrler B, Gélineas S, Böhm ML, Walker BJ et al. Resonant energy transfer of triplet excitons from pentacene to PbSe nanocrystals. Nat Mater 2014; 13: 1033–1038.
Thompson NJ, Wilson MWB, Congreve DN, Brown PR, Scherer JM et al. Energy harvesting of non-emissive triplet excitons in tetracene by emissive PbS nanocrystals. Nat Mater 2014; 13: 1039–1043.
Abrams ZR, Charghi M, Niv A, Gladden C, Zhang X . Theoretical efficiency of 3rd generation solar cells: comparison between carrier multiplication and down-conversion. Sol Energy Mater Sol Cells 2012; 99: 308–315.
Naczynski DJ, Tan MC, Zevon M, Wall B, Kohl J et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat Commun 2013; 4: 2199.
Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV . Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J Phys D: Appl Phys 2005; 38: 2543–2555.
Eick HA . The preparation, lattice parameters and some chemical properties of the rare earth mono-thio oxides. J Am Chem Soc 1958; 80: 43–44.
Martín-Rodríguez R, Fischer S, Ivaturi A, Froehlich B, Krämer KW et al. Highly efficient IR to NIR upconversion in Gd2O2S:Er3+ for photovoltaic applications. Chem Mater 2013; 25: 1912–1921.
Yi Q, Tsuboi T, Zhou S, Nakai Y, Lin H et al. Investigation of emission properties of Tm3+:Y2O3 transparent ceramic. Chin Opt Lett 2012; 10: 091602.
Rabouw FT, den Hartog SA, Senden T, Meijerink A . Photonic effects on the Förster resonance energy transfer efficiency. Nat Commun 2014; 5: 3610.
Yu DC, Rabouw FT, Boon WQ, Kieboom T, Ye S et al. Insights into the energy transfer mechanism in Ce3+-Yb3+ codoped YAG phosphors. Phys Rev B 2014; 90: 165126.
Yu DC, Ye S, Peng MY, Zhang QY, Wondraczek L . Sequential three-step three-photon near-infrared quantum splitting in β-NaYF4:Tm3+. Appl Phys Lett 2012; 100: 191911.
O’Connor R, Mahiou R, Martinant D, Fournier MT . Luminescence and dynamics in Tm3+:NaYF4 . J Alloys Compd 1995; 225: 107–110.
ten Kate OM, van der Kolk E . Quantum tripling in Tm3+ doped La2BaZnO5 phosphors for efficiency enhancement of small bandgap solar cells. J Lumin 2014; 156: 262–265.
Thrash RJ, Johnson LF . Upconversion laser emission from Yb3+-sensitized Tm3+ in BaY2F8 . J Opt Soc Am B 1994; 11: 881–885.
Zhao JB, Jin DY, Schartner EP, Lu YQ, Liu YJ et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat Nanotechnol 2013; 8: 729–734.
Wang F, Deng RR, Wang J, Wang QX, Han Y et al. Tuning upconversion through energy migration in core-shell nanoparticles. Nat Mater 2011; 10: 968–973.
Wang YZ, Yu DC, Lin HH, Ye S, Peng MY et al. Broadband three-photon near-infrared quantum cutting in Tm3+ singly doped YVO4 . J Appl Phys 2013; 114: 203510.
Chen XB, Li S, Salamo GJ, Li YL, He LZ et al. Sensitized intense near-infrared downconversion quantum cutting three-photon luminescence phenomena of the Tm3+ ion activator in Tm3+Bi3+:YNbO4 powder phosphor. Opt Express 2015; 23: A51–A61.
Dong SL, Lin HH, Yu T, Zhang QY . Near-infrared quantum-cutting luminescence and energy transfer properties of Ca3(PO4)2:Tm3+,Ce3+ phosphors. J Appl Phys 2014; 116: 023517.
Zhang GG, Liu CM, Wang J, Kuang XJ, Su Q . A dual-mode solar spectral converter CaLaGa3S6O:Ce3+,Pr3+: UV-Vis-NIR luminescence properties and solar spectral converting mechanism. J Mater Chem 2012; 22: 2226–2232.
Auzel F, Baldacchini G, Laversenne L, Boulon G . Radiation trapping and self-quenching analysis in Yb3+, Er3+, and Ho3+ doped Y2O3 . Opt Mater 2003; 24: 103–109.
Martín-Rodríguez R, Rabouw FT, Trevisani M, Bettinelli M, Meijerink A . Upconversion dynamics in Er3+-doped Gd2O2S: influence of excitation power, Er3+ concentration, and defects. Adv Optical Mater 2015; 3: 558–567.
Acknowledgements
Financial support from the National Science Foundation of China (51125005 and 51472088) is gratefully acknowledged. Dechao Yu thanks the China Scholarship Council (CSC, File No. 201206150022) for a scholarship. This work is part of the research program of the ‘Stichting voor Fundamenteel Onderzoek der Materie (FOM)’, which is financially supported by the ‘Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)’.
Note: Accepted article preview online 20 June 2015
Author information
Authors and Affiliations
Corresponding authors
Additional information
Note: Supplementary information for this article can be found on the Light: Science & Applications' website.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Yu, DC., Martín-Rodríguez, R., Zhang, QY. et al. Multi-photon quantum cutting in Gd2O2S:Tm3+ to enhance the photo-response of solar cells. Light Sci Appl 4, e344 (2015). https://doi.org/10.1038/lsa.2015.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/lsa.2015.117
Keywords
This article is cited by
-
Intervalence charge transfer of Cr3+-Cr3+ aggregation for NIR-II luminescence
Light: Science & Applications (2023)
-
Near-infrared quantum cutting luminescence in Pr3+/Yb3+ doped lead bismuth borate glass
Scientific Reports (2022)
-
Crystal structure, infrared luminescence and magnetic properties of Tm3+-doped and Tm3+-, Dy3+-codoped BaY2Ge3O10 germanates
Journal of Materials Science: Materials in Electronics (2021)
-
Understanding and tuning blue-to-near-infrared photon cutting by the Tm3+/Yb3+ couple
Light: Science & Applications (2020)
-
Development of highly luminescent PMMA films doped with Eu3+β-diketonate coordinated on ancillary ligand
Journal of Materials Science: Materials in Electronics (2019)