Abstract
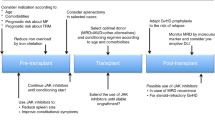
The myeloproliferative neoplasm, myelofibrosis (MF), has only one therapeutic intervention that is potentially curative in these individuals, specifically that of allogeneic stem cell transplantation (ASCT). ASCT has been utilized up to this juncture, primarily in younger individuals with higher risk disease. There is more limited data on outcomes in individuals over the age of 60 years. The choice of an individualized therapeutic intervention for a patient with MF is a very complex issue and is dependent on several factors. The first factor being their overall prognosis with their illness (which can vary from a median of 2 years in high-risk patients to over 10 years in low-risk patients) and the potential impact of a therapeutic intervention not only on survival but also on quality of life. Current available therapies have been strictly palliative for disease-associated anemia and/or splenomegaly. At present, we have a new generation of inhibitors of JAK2 (Ruxolitinib, CYT387, SB1518, TG101348, with others in development), which have been shown to improve splenomegaly, improve symptomatic burden of illness and improve quality of life. In addition, these inhibitors of JAK2 may have an impact on the natural history of MF, but confirmation of the presence and degree of this impact is still pending. Clinical availability of JAK2 inhibitors may alter the timing of transplant in marginal transplant candidates (that is, those over the age of 60), may have a role preceding ASCT to improve spleen size and performance status before transplant and might be frontline therapy in intermediate and high-risk patients who are not candidates for ASCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mesa RA, Green A, Barosi G, Verstovsek S, Vardiman J, Gale RP . MPN-associated myelofibrosis (MPN-MF). Leukemia Res 2011; 35: 12–13.
Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 2010; 16: 358–367.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010; 115: 1703–1708.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011; 29: 392–397.
Samuelson S, Sandmaier BM, Heslop HE, Popat U, Carrum G, Champlin RE et al. Allogeneic haematopoietic cell transplantation for myelofibrosis in 30 patients 60–78 years of age. Br J Haematol 2011; 153: 76–82.
Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant 2010; 45: 458–463.
Ditschkowski M, Elmaagacli AH, Trenschel R, Steckel NK, Koldehoff M, Beelen DW . No influence of V617F mutation in JAK2 on outcome after allogeneic hematopoietic stem cell transplantation (HSCT) for myelofibrosis. Biol Blood Marrow Transplant 2006; 12: 1350–1351.
Kroger N, Badbaran A, Holler E, Hahn J, Kobbe G, Bornhäuser M et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood 2007; 109: 1316–1321.
Alchalby H, Lioznov M, Fritzsche-Friedland U, Badbaran A, Zabelina T, Bacher U et al. Circulating CD34(+) cells as prognostic and follow-up marker in patients with myelofibrosis undergoing allo-SCT. Bone Marrow Transplantation 2012; 47: 143–145.
Kroger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009; 114: 5264–5270.
Alchalby H, Yunus D, Kobbe G, Holler E, Schwerdtfeger R . Risk Models Predicting Survival after Reduced Intensity Transplantation for Myelofibrosis. Br J Haematol 2011 (in press).
Scott BL, Gooley Ted A, Sorror ML, Rezvani AR, Linenberger L et al. The dynamic international prognostic scoring system for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood 2011 (in press).
Zihai Li, Gooley Ted A, Appelbaum Frederick R, Deeg Joachim . Splenectomy and hemopoietic stem cell transplantation for myelofibrosis. Blood 2001; 97: 2180–2181.
Stewart WA, Pearce R, Kirkland KE, Bloor A, Thomson K, Apperley J . The role of allogeneic SCT in primary myelofibrosis: A British society for blood and marrow transplantation study. Bone Marrow Transplant 2010; 45: 1587–1593.
Guardiola P, Anderson JE, Bandini G, Cervantes F, Runde V, Arcese W et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood 1999; 93: 2831–2838.
Robin M, Espérou H, de Latour RP, Petropoulou AD, Xhaard A, Ribaud P et al. Splenectomy after allogeneic haematopoietic stem cell transplantation in patients with primary myelofibrosis. Br J Haematol 2010; 150: 721–724.
Mesa RA, Nagorney DS, Schwager S, Allred J, Tefferi A . Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer 2006; 107: 361–370.
Li Z, Gooley T, Applebaum FR, Deeg HJ . Splenectomy and hemopoietic stem cell transplantation for myelofibrosis. Blood 2001; 97: 2180–2181.
Mesa RA . How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 2009; 113: 5394–5400.
Huang J, Tefferi A . Erythropoiesis stimulating agents have limited therapeutic activity in transfusion-dependent patients with primary myelofibrosis regardless of serum erythropoietin level. Eur J Haematol 2009; 83: 154–155.
Cervantes F, Hernandez-Boluda JC, Alvarez A, Nadal E, Montserrat E . Danazol treatment of idiopathic myelofibrosis with severe anemia. Haematologica 2000; 85: 595–599.
Mesa RA, Steensma DP, Pardanani A, Li CY, Elliott M, Kaufmann SH et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood 2003; 101: 2534–2541.
Tefferi A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN . Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood 2000; 95: 2226–2233.
Elliott MA, Chen MG, Silverstein MN, Tefferi A . Splenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasia. Br J Haematol 1998; 103: 505–511.
Tefferi A, Cortes J, Verstovsek S, Mesa RA, Thomas D, Lasho TL et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood 2006; 108: 1158–1164.
Faoro LN, Tefferi A, Mesa RA . Long-term analysis of the palliative benefit of 2-chlorodeoxyadenosine for myelofibrosis with myeloid metaplasia. Eur J Haematol 2005; 74: 117–120.
Begna KH, Mesa RA, Pardanani A, Hogan WJ, Litzow MR, McClure RF et al. A phase-2 trial of low-dose pomalidomide in myelofibrosis. Leukemia 2011; 25: 301–304.
Verstovsek S, Mesa RA, Gotlib JR, Levy RS, Gupts V, DiPersio J et al. Results of COMFORT-I, a randomized double-blind phase III trial of JAK 1/2 inhibitor INCB18424 (424) versus placebo (PB) for patients with myelofibrosis (MF). ASCO Meeting Abstracts 2011; 29: 6500.
Harrison CN, Kiladjian J, Al-Ali HK, Gisslinger H, Waltzman RJ, Stalbovskaya V et al. Results of a randomized study of the JAK inhibitor INC424 compared with best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera-myelofibrosis (PPV-MF) or post-essential thrombocythemia myelofibrosis (PET-MF). ASCO Meeting Abstracts 2011; 29: LBA6501.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Pardanani AD, Caramazza G, George G, Lasho TL, Hogan WJ, Litzow MR et al. Safety and efficacy of CYT387, a JAK-1/2 inhibitor, for the treatment of myelofibrosis. ASCO Meeting Abstracts 2011; 29: 6514.
Deeg JH, Odenkio O, Scott BL, Estrov Z, Cortes JE, Thomas DA et al. Phase II study of SB1518, an orally available novel JAK2 inhibitor, in patients with myelofibrosis. ASCO Meeting Abstracts 2011; 29: 6515.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A . Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 2011; 29: 1356–1363.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232.
Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system [see comments]. Blood 1996; 88: 1013–1018.
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901.
Kerbauy DM, Gooley TA, Sale GE, Flowers ME, Doney KC, Georges GE et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 2007; 13: 355–365.
Patriarca F, Bacigalupo A, Sperotto A, Isola M, Bruno B, van Lint MT et al. Outcome of allogeneic stem cell transplantation following reduced-intensity conditioninig regimen in patients with idiopathic myelofibrosis: the g.I.T.m.o. Experience. Mediterr J Hematol Infect Dis 2010; 2: e2010010.
Stewart WA, Pearce R, Kirkland KE, Bloor A, Thomson K, Apperley J et al. The role of allogeneic SCT in primary myelofibrosis: a British Society for Blood and Marrow Transplantation study. Bone Marrow Transplant 2010; 45: 1587–1593.
Merup M, Lazarevic V, Nahi H, Andreasson B, Malm C, Nilsson L et al. Different outcome of allogeneic transplantation in myelofibrosis using conventional or reduced-intensity conditioning regimens. Br J Haematol 2006; 135: 367–373.
Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood 2005; 105: 4115–4119.
Snyder DS, Palmer J, Gaal K, Stein AS, Pullarkat V, Sahebi F et al. Improved outcomes using tacrolimus/sirolimus for graft-versus-host disease prophylaxis with a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transplant 2006; 16: 281–286.
Hexner E, Goldberg JD, Prchal JT, Demakos EP, Swierczek S, Weinberg RS et al. A Multicenter, Open Label Phase I/II Study of CEP701 (Lestaurtinib) in adults with myelofibrosis; a Report On Phase I: A Study of the Myeloproliferative Disorders Research Consortium (MPD-RC). Blood 2009; 114: A754.
Moliterno AR, Hexner E, Roboz GJ, Carroll M, Luger S, Mascarenhas J et al. An Open-Label Study of CEP-701 in patients with JAK2 V617F-positive PV and ET: update of 39 enrolled patients. Blood 2009; 114: A753.
Ma L, Zhao B, Walgren R, Clayton JA, Blosser WD, Burkholder TP et al. Efficacy of LY2784544, a small molecule inhibitor selective for mutant JAK2 kinase, in JAK2 V617F-induced hematologic malignancy models. Blood 2010; 116: 4087.
Florensa L, Bellosillo B, Arenillas L, Ma L, Walgren R, Alvarez A et al. LY2784544, a novel JAK2 inhibitor, decreases in vitro growth of hematopoietic human progenitors from JAK2 V617F positive polycythemia vera patients. Blood 2010; 116: 5054.
Shide K, Nakaya Y, Kameda T, Shimoda H, Hidaka T, Kubuki Y et al. NS-018, a potent novel JAK2 inhibitor, effectively treats murine mpn induced by the Janus Kinase 2 (JAK2) V617F mutant. Blood 2010; 116: 4106.
Nakaya Y, Naito H, Homan J, Sugahara S, Horio T, Niwa T et al. Preferential inhibition of an activated form of Janus Kinase 2 (JAK2) by a Novel JAK2 inhibitor, NS-018. Blood 2010; 116: 4107.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fauble, V., Leis, J. & Mesa, R. Allogeneic stem cell transplant for myelofibrosis patients over age 60: likely impact of the JAK2 inhibitors. Leukemia Suppl 1 (Suppl 1), S2–S7 (2012). https://doi.org/10.1038/leusup.2012.2
Published:
Issue Date:
DOI: https://doi.org/10.1038/leusup.2012.2
Keywords
This article is cited by
-
Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation
Annals of Hematology (2017)
-
The New Landscape of Therapy for Myelofibrosis
Current Hematologic Malignancy Reports (2013)