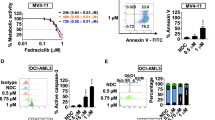
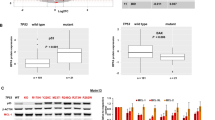
Abstract
CXCR4 is a key player in the retention and survival of human acute myeloid leukemia (AML) blasts in the bone marrow (BM) microenvironment. We studied the effects of the CXCR4 antagonist BL-8040 on the survival of AML blasts, and investigated the molecular mechanisms by which CXCR4 signaling inhibition leads to leukemic cell death. Treatment with BL-8040 induced the robust mobilization of AML blasts from the BM. In addition, AML cells exposed to BL-8040 underwent differentiation. Furthermore, BL-8040 induced the apoptosis of AML cells in vitro and in vivo. This apoptosis was mediated by the upregulation of miR-15a/miR-16-1, resulting in downregulation of the target genes BCL-2, MCL-1 and cyclin-D1. Overexpression of miR-15a/miR-16-1 directly induced leukemic cell death. BL-8040-induced apoptosis was also mediated by the inhibition of survival signals via the AKT/ERK pathways. Importantly, treatment with a BCL-2 inhibitor induced apoptosis and act together with BL-8040 to enhance cell death. BL-8040 also synergized with FLT3 inhibitors to induce AML cell death. Importantly, this combined treatment prolonged the survival of tumor-bearing mice and reduced minimal residual disease in vivo. Our results provide a rationale to test combination therapies employing BL-8040 and BCL-2 or FLT3 inhibitors to achieve increased efficacy of these agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peled A, Tavor S . Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics 2013; 3: 34–39.
Estey E, Dohner H . Acute myeloid leukaemia. Lancet 2006; 368: 1894–1907.
Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 2003; 9: 1158–1165.
Ayala F, Dewar R, Kieran M, Kalluri R . Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 2009; 23: 2233–2241.
Burger JA, Peled A . CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 2009; 23: 43–52.
Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE . Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood 2004; 104: 550–557.
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009; 113: 6206–6214.
Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood 2009; 113: 6215–6224.
Cho BS, Zeng Z, Mu H, Wang Z, Konoplev S, McQueen T et al. Antileukemia activity of the novel peptidic CXCR4 antagonist LY2510924 as monotherapy and in combination with chemotherapy. Blood 2015; 126: 222–232.
Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C et al. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res 2013; 19: 357–366.
Peng SB, Zhang X, Paul D, Kays LM, Ye M, Vaillancourt P et al. Inhibition of CXCR4 by LY2624587, a fully humanized anti-CXCR4 antibody induces apoptosis of hematologic malignancies. PLoS ONE 2016; 11: e0150585.
Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett 2004; 569: 99–104.
Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen X . PET of tumor CXCR4 expression with 4-18 F-T140. J Nucl Med 2010; 51: 1796–1804.
Jacobson O, Weiss ID, Szajek LP, Niu G, Ma Y, Kiesewetter DO et al. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics 2011; 1: 251–262.
Abraham M, Biyder K, Begin M, Wald H, Weiss ID, Galun E et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4 F-benzoyl-TN14003. Stem Cells 2007; 25: 2158–2166.
Peled A, Abraham M, Avivi I, Rowe JM, Beider K, Wald H et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin Cancer Res 2014; 20: 469–479.
Abraham M, Weiss ID, Wald H, Wald O, Nagler A, Beider K et al. Sequential administration of the high affinity CXCR4 antagonist BKT140 promotes megakaryopoiesis and platelet production. Br J Haematol 2013; 163: 248–259.
Beider K, Begin M, Abraham M, Wald H, Weiss ID, Wald O et al. CXCR4 antagonist 4 F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol 2011; 39: 282–292.
Beider K, Ribakovsky E, Abraham M, Wald H, Weiss L, Rosenberg E et al. Targeting the CD20 and CXCR4 pathways in non-Hodgkin lymphoma with rituximab and high-affinity CXCR4 antagonist BKT140. Clin Cancer Res 2013; 19: 3495–3507.
Beider K, Darash-Yahana M, Blaier O, Koren-Michowitz M, Abraham M, Wald H et al. Combination of imatinib with CXCR4 antagonist BKT140 overcomes the protective effect of stroma and targets CML in vitro and in vivo. Mol Cancer Ther 2014; 13: 1155–1169.
Fahham D, Weiss ID, Abraham M, Beider K, Hanna W, Shlomai Z et al. In vitro and in vivo therapeutic efficacy of CXCR4 antagonist BKT140 against human non-small cell lung cancer. J Thorac Cardiovasc Surg 2012; 144: 1167–1175.
Kelly PN, Strasser A . The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 2011; 18: 1414–1424.
Vaux DL, Cory S, Adams JM . Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988; 335: 440–442.
Pekarsky Y, Croce CM . Role of miR-15/16 in CLL. Cell Death Differ 2015; 22: 6–11.
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005; 102: 13944–13949.
Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 2008; 14: 1271–1277.
Gao SM, Yang J, Chen C, Zhang S, Xing CY, Li H et al. miR-15a/16-1 enhances retinoic acid-mediated differentiation of leukemic cells and is up-regulated by retinoic acid. Leuk Lymphoma 2011; 52: 2365–2371.
Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia 2005; 19: 1543–1549.
Quentmeier H, Reinhardt J, Zaborski M, Drexler HG . FLT3 mutations in acute myeloid leukemia cell lines. Leukemia 2003; 17: 120–124.
Pratz KW Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood 2009; 113: 3938–3946.
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 2004; 103: 3669–3676.
Xiaochuan Yang AS, Levis MJ . Persistent ERK activation in bone marrow blasts may account for the difference in bone marrow versus peripheral blood response to FLT3 inhibition in FLT3/ITD AML. Blood 2011; 118: 736.
De Kouchkovsky I, Abdul-Hay M . Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016; 6: e441.
Gao SM, Xing CY, Chen CQ, Lin SS, Dong PH, Yu FJ . miR-15a and miR-16-1 inhibit the proliferation of leukemic cells by down-regulating WT1 protein level. J Exp Clin Cancer Res 2011; 30: 110.
Gao SM, Yang JJ, Chen CQ, Chen JJ, Ye LP, Wang LY et al. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J Exp Clin Cancer Res 2012; 31: 27.
Cantley LC . The phosphoinositide 3-kinase pathway. Science 2002; 296: 1655–1657.
Mo W, Chen J, Patel A, Zhang L, Chau V, Li Y et al. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell 2013; 152: 1077–1090.
Fathi AT, Grant S, Karp JE . Exploiting cellular pathways to develop new treatment strategies for AML. Cancer Treat Rev 2010; 36: 142–150.
Rahmani M, Aust MM, Attkisson E, Williams DC Jr, Ferreira-Gonzalez A, Grant S . Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 2012; 119: 6089–6098.
Schimmer AD . Novel therapies targeting the apoptosis pathway for the treatment of acute myeloid leukemia. Curr Treat Options Oncol 2007; 8: 277–286.
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 2014; 4: 362–375.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208.
Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 2015; 6: e1593.
Niu X, Wang G, Wang Y, Caldwell JT, Edwards H, Xie C et al. Acute myeloid leukemia cells harboring MLL fusion genes or with the acute promyelocytic leukemia phenotype are sensitive to the Bcl-2-selective inhibitor ABT-199. Leukemia 2014; 28: 1557–1560.
Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res 2016; 22: 4440–4451.
Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep 2016; 6: 27696.
Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia 2008; 22: 2151–5158.
Peled A, Wald O, Burger J . Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs 2012; 21: 341–353.
Zhang Y, Patel S, Abdelouahab H, Wittner M, Willekens C, Shen S et al. CXCR4 inhibitors selectively eliminate CXCR4-expressing human acute myeloid leukemia cells in NOG mouse model. Cell Death Dis 2012; 3: e396.
Fukuda S, Broxmeyer HE, Pelus LM . Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood 2005; 105: 3117–3126.
Onishi C, Mori-Kimachi S, Hirade T, Abe M, Taketani T, Suzumiya J et al. Internal tandem duplication mutations in FLT3 gene augment chemotaxis to Cxcl12 protein by blocking the down-regulation of the Rho-associated kinase via the Cxcl12/Cxcr4 signaling axis. J Biol Chem 2014; 289: 31053–31065.
Grundler R, Brault L, Gasser C, Bullock AN, Dechow T, Woetzel S et al. Dissection of PIM serine/threonine kinases in FLT3-ITD-induced leukemogenesis reveals PIM1 as regulator of CXCL12-CXCR4-mediated homing and migration. J Exp Med 2009; 206: 1957–1970.
Kim KT, Carroll AP, Mashkani B, Cairns MJ, Small D, Scott RJ . MicroRNA-16 is down-regulated in mutated FLT3 expressing murine myeloid FDC-P1 cells and interacts with Pim-1. PLoS ONE 2012; 7: e44546.
Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 2008; 22: 808–818.
Abraham M, Beider K, Wald H, Weiss ID, Zipori D, Galun E et al. The CXCR4 antagonist 4 F-benzoyl-TN14003 stimulates the recovery of the bone marrow after transplantation. Leukemia 2009; 23: 1378–1388.
Acknowledgements
This work was supported by grants from Biokine Therapeutics and BiolineRX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Michal Abraham, Hanna Wald and Orly Eizenberg are employees and shareholders of Biokine Therapeutics; Amnon Peled serves as a consultant for Biokine Therapeutics and is also a shareholder. Yaron Pereg is an employee of BioLineRx. Remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Abraham, M., Klein, S., Bulvik, B. et al. The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression. Leukemia 31, 2336–2346 (2017). https://doi.org/10.1038/leu.2017.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.82
This article is cited by
-
Homoharringtonine enhances cytarabine-induced apoptosis in acute myeloid leukaemia by regulating the p38 MAPK/H2AX/Mcl-1 axis
BMC Cancer (2024)
-
Host-derived growth factors drive ERK phosphorylation and MCL1 expression to promote osteosarcoma cell survival during metastatic lung colonization
Cellular Oncology (2024)
-
Targeting Pim kinases in hematological cancers: molecular and clinical review
Molecular Cancer (2023)
-
CXC chemokine receptor 4 (CXCR4) blockade in cancer treatment
Journal of Cancer Research and Clinical Oncology (2023)
-
The upregulation of stromal antigen 3 expression suppresses the phenotypic hallmarks of hepatocellular carcinoma through the Smad3-CDK4/CDK6-cyclin D1 and CXCR4/RhoA pathways
BMC Gastroenterology (2022)