Abstract
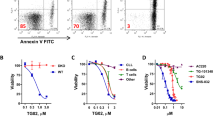
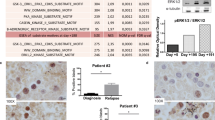
Systemic mastocytosis (SM) is a mast cell (MC) neoplasm with complex pathology and a variable clinical course. In aggressive SM (ASM) and MC leukemia (MCL), responses to conventional drugs are poor and the prognosis is dismal. R763 is a multi-kinase inhibitor that blocks the activity of Aurora-kinase-A/B, ABL1, AKT and FLT3. We examined the effects of R763 on proliferation and survival of neoplastic MC. R763 produced dose-dependent inhibition of proliferation in the human MC lines HMC-1.1 (IC50 5–50 nM), HMC-1.2 (IC50 1–10 nM), ROSAKIT WT (IC50 1–10 nM), ROSAKIT D816V (IC50 50–500 nM) and MCPV-1.1 (IC50 100–1000 nM). Moreover, R763 induced growth inhibition in primary neoplastic MC in patients with ASM and MCL. Growth-inhibitory effects of R763 were accompanied by signs of apoptosis and a G2/M cell cycle arrest. R763 also inhibited phosphorylation of KIT, BTK, AKT and STAT5 in neoplastic MC. The most sensitive target appeared to be STAT5. In fact, tyrosine phosphorylation of STAT5 was inhibited by R763 at 10 nM. At this low concentration, R763 produced synergistic growth-inhibitory effects on neoplastic MC when combined with midostaurin or dasatinib. Together, R763 is a novel promising multi-kinase inhibitor that blocks STAT5 activation and thereby overrides drug-resistance in neoplastic MC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Metcalfe DD . Classification and diagnosis of mastocytosis: current status. J Invest Dermatol 1991; 96: 2S–4S.
Valent P . Biology, classification and treatment of human mastocytosis. Wien Klin Wochenschr 1996; 108: 385–397.
Horny HP, Valent P . Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res 2001; 25: 543–551.
Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD . Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol 2002; 81: 677–690.
Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol 2003; 122: 695–717.
Akin C, Metcalfe DD . Systemic mastocytosis. Annu Rev Med 2004; 55: 419–432.
Pardanani A, Tefferi A . Systemic mastocytosis in adults: a review on prognosis and treatment based on 342 Mayo Clinic patients and current literature. Curr Opin Hematol 2010; 17: 125–132.
Valent P, Ghannadan M, Akin C, Krauth MT, Selzer E, Mayerhofer M et al. On the way to targeted therapy of mast cell neoplasms: identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur J Clin Invest 2004; 34 (Suppl 2): 41–52.
Tefferi A, Pardanani A . Clinical genetic, and therapeutic insights into systemic mast cell disease. Curr Opin Hematol 2004; 11: 58–64.
Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA 1995; 92: 10560–10564.
Feger F, Ribadeau Dumas A, Leriche L, Valent P, Arock M . Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol 2002; 127: 110–114.
Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG et al. Somatic c-kit activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996; 12: 312–314.
Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol 2001; 113: 357–364.
Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia 2015; 29: 1223–1232.
Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of the c-kit product. J Clin Invest 1993; 92: 1736–1744.
Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C . Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 2006; 108: 286–291.
Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol 2003; 31: 686–692.
Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica 2007; 92: 1451–1459.
Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood 2006; 107: 752–759.
Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood 2005; 106: 2865–2870.
Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med 2016; 374: 2530–2541.
Gleixner KV, Mayerhofer M, Cerny-Reiterer S, Hörmann G, Rix U, Bennett KL et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and BTK activation and disruption by dasatinib and bosutinib. Blood 2011; 118: 1885–1898.
Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica 2011; 96: 459–463.
Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 2013; 122: 2460–2466.
Bibi S, Langenfeld F, Jeanningros S, Brenet F, Soucie E, Hermine O et al. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol Allergy Clin North Am 2014; 34: 239–262.
Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS ONE 2014; 9: e85362.
Baumgartner C, Cerny-Reiterer S, Sonneck K, Mayerhofer M, Gleixner KV, Fritz R et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis: subcellular distribution and role of the transforming oncoprotein KIT D816V. Am J Pathol 2009; 175: 2416–2429.
Chaix A, Lopez S, Voisset E, Gros L, Dubreuil P, De Sepulveda P . Mechanisms ofSTAT protein activation by oncogenic KIT mutants in neoplastic mast cells. J Biol Chem 2011; 286: 5956–5966.
Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signalling cascade. Blood 2008; 112: 2463–2473.
Farag SS . The potential role of Aurora kinase inhibitors in haematological malignancies. Br J Haematol 2011; 155: 561–579.
Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM . Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res 2006; 16: 356–366.
Lok W, Klein RQ, Saif MW . Aurora kinase inhibitors as anti-cancer therapy. Anticancer Drugs 2010; 21: 339–350.
McLaughlin J, Markovtsov V, Li H, Wong S, Gelman M, Zhu Y et al. Preclinical characterization of Aurora kinase inhibitor R763 identified through an image-based phenotypic screen. J Cancer Res Clin Oncol 2010; 136: 99–113.
Cicenas J . The Aurora kinase inhibitors in cancer research and therapy. J Cancer Res Clin Oncol 2016; 142: 1995–2012.
Valent P, Horny H-P, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Conference Report of ‘Year 2000 Working Conference on Mastocytosis’ Leuk Res 2001; 25: 603–625.
Valent P, Akin C, Metcalfe DD . Mastocytosis: 2016-updated WHO classification and novel emerging treatment concepts. Blood 2017; 129: 1420–1427.
Butterfield JH, Weiler D, Dewald G, Gleich GJ . Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res 1988; 12: 345–355.
Saleh R, Wedeh G, Herrmann H, Bibi S, Cerny-Reiterer S, Sadovnik I et al. A new human mast cell line expressing a functional IgE receptor converts to factor-independence and tumorigenicity by KIT D816V-transfection. Blood 2014; 124: 111–120.
Hoermann G, Blatt K, Greiner G, Putz EM, Berger A, Herrmann H et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J 2014; 28: 3540–3551.
DeVinney R, Gold WM . Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol 1990; 3: 413–420.
Hadzijusufovic E, Peter B, Herrmann H, Rülicke T, Cerny-Reiterer S, Schuch K, Kenner L, Thaiwong T, Yuzbasiyan-Gurkan V, Pickl WF, Willmann M, Valent P . NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy 2012; 67: 858–868.
Peter B, Gleixner KV, Cerny-Reiterer S, Herrmann H, Winter V, Hadzijusufovic E et al. Polo-like kinase-1 as novel target in neoplastic mast cells: demonstration of growth-inhibitory effects of siRNA and the Polo-like kinase-1 targeting drug BI 2536. Haematologica 2011; 96: 672–680.
Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN . Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity 1999; 11: 225–230.
Friedbichler K, Themanns M, Mueller KM, Schlederer M, Kornfeld JW, Terracciano LM et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology 2012; 55: 941–952.
Wedeh G, Cerny-Reiterer S, Eisenwort G, Herrmann H, Blatt K, Hadzijusufovic E et al. Identification of bromodomain-containing protein-4 as a novel marker and epigenetic target in mast cell leukemia. Leukemia 2015; 29: 2230–2237.
Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 2013; 5: 1704–1713.
Hoermann G, Cerny-Reiterer S, Herrmann H, Blatt K, Bilban M, Gisslinger H et al. Identification of oncostatin M as a JAK2 V617F-dependent amplifier of cytokineproduction and bone marrow remodeling in myeloproliferative neoplasms. FASEB J 2012; 26: 894–906.
Keller A, Wingelhofer B, Peter B, Bauer K, Berger D, Gamperl S et al. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet Comp Oncol 2017; epub ahead of print 11 April 2017 doi:10.1111/vco.12311.
Peter B, Winter GE, Blatt K, Bennett KL, Stefanzl G, Rix U et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia 2016; 30: 464–472.
Tobío A, Alfonso A, Fernández-Araujo A, Alonso E, Botana LM . Protein kinase C modulates Aurora-kinase inhibition induced by CCT129202 in HMC-1560, 816 cell line. Antiinflamm Antiallergy Agents Med Chem 2013; 12: 265–276.
Acknowledgements
This study was supported by Austrian Science Fund (FWF), SFB grants F4701-B20, F4704-B20, F4707-B20 and F4710-B20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
GH received honoraria from Novartis and Ariad. WRS received honoraria from Novartis and Celgene, and a research grant from Lipomed. PV, H-PH and AR served as a Consultant in a global Novartis trial examining the effects of midostaurin in advanced SM. JZ received institutional support from Boehringer-Ingelheim, and is consultant and stock holder at Mirimus Inc. AR received a research grant from Novartis, honoraria from Novartis and BMS, and served in advisory boards organized by Novartis. MA received research grants from Blueprint and Deciphera, and received honoraria from Deciphera. PV received research grants from Novartis, Blueprint and Deciphera, and honoraria from Novartis, Celgene, Pfizer and Deciphera. The remaining authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Peter, B., Bibi, S., Eisenwort, G. et al. Drug-induced inhibition of phosphorylation of STAT5 overrides drug resistance in neoplastic mast cells. Leukemia 32, 1016–1022 (2018). https://doi.org/10.1038/leu.2017.338
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.338
This article is cited by
-
The two sides of chromosomal instability: drivers and brakes in cancer
Signal Transduction and Targeted Therapy (2024)
-
SETD2 non genomic loss of function in advanced systemic mastocytosis is mediated by an Aurora kinase A/MDM2 axis and can be therapeutically targeted
Biomarker Research (2023)
-
Gab2 deficiency prevents Flt3-ITD driven acute myeloid leukemia in vivo
Leukemia (2022)
-
Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide
Oncogene (2018)