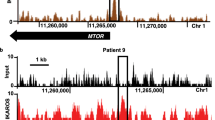
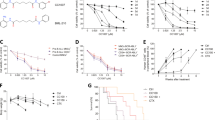
Abstract
Although substantial progress has been made in the treatment of B-cell acute lymphoblastic leukemia (B-ALL), the prognosis of patients with either refractory or relapsed B-ALL remains dismal. Novel therapeutic strategies are needed to improve the outcome of these patients. KPT-9274 is a novel dual inhibitor of p21-activated kinase 4 (PAK4) and nicotinamide phosphoribosyltransferase (NAMPT). PAK4 is a serine/threonine kinase that regulates a variety of fundamental cellular processes. NAMPT is a rate-limiting enzyme in the salvage biosynthesis pathway of nicotinamide adenine dinucleotide (NAD) that plays a vital role in energy metabolism. Here, we show that KPT-9274 strongly inhibits B-ALL cell growth regardless of cytogenetic abnormalities. We also demonstrate the potent in vivo efficacy and tolerability of KPT-9274 in a patient-derived xenograft murine model of B-ALL. Interestingly, although KPT-9274 is a dual PAK4/NAMPT inhibitor, B-ALL cell growth inhibition by KPT-9274 was largely abolished with nicotinic acid supplementation, indicating that the inhibitory effects on B-ALL cells are mainly exerted by NAD+ depletion through NAMPT inhibition. Moreover, we have found that the extreme susceptibility of B-ALL cells to NAMPT inhibition is related to the reduced cellular NAD+ reserve. NAD+ depletion may be a promising alternative approach to treating patients with B-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pui C-H, Relling MV, Downing JR . Acute lymphoblastic leukemia. N Engl J Med 2004; 350: 1535–1548.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004; 101: 2788–2801.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109: 944–950.
Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol 2010; 28: 3644–3652.
Soverini S, De Benedittis C, Machova Polakova K, Brouckova A, Horner D, Iacono M et al. Unraveling the complexity of tyrosine kinase inhibitor-resistant populations by ultra-deep sequencing of the BCR-ABL kinase domain. Blood 2013; 122: 1634–1648.
Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell 2014; 26: 428–442.
Warburg O, Wind F, Negelein E . The metabolism of tumors in the body. J Gen Physiol 1927; 8: 519–530.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012; 483: 474–478.
Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev 2012; 26: 1326–1338.
Yang M, Soga T, Pollard PJ, Adam J . The emerging role of fumarate as an oncometabolite. Front Oncol 2012; 2: 85.
Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell 2015; 28: 773–784.
Cantó C, Menzies KJ, Auwerx J . NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 2015; 22: 31–53.
Sampath D, Zabka TS, Misner DL, O’Brien T, Dragovich PS . Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther 2015; 151: 16–31.
Cea M, Cagnetta A, Fulciniti M, Tai Y-T, Hideshima T, Chauhan D et al. Targeting NAD+ salvage pathway induces autophagy in multiple myeloma cells via mTORC1 and extracellular signal-regulated kinase (ERK1/2) inhibition. Blood 2012; 120: 3519–3529.
Chini CCS, Guerrico AMG, Nin V, Camacho-Pereira J, Escande C, Barbosa MT et al. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res 2014; 20: 120–130.
Gehrke I, Bouchard EDJ, Beiggi S, Poeppl AG, Johnston JB, Gibson SB et al. On-target effect of FK866, a nicotinamide phosphoribosyl transferase inhibitor, by apoptosis-mediated death in chronic lymphocytic leukemia cells. Clin Cancer Res 2014; 20: 4861–4872.
Matheny CJ, Wei MC, Bassik MC, Donnelly AJ, Kampmann M, Iwasaki M et al. Next-generation NAMPT inhibitors identified by sequential high-throughput phenotypic chemical and functional genomic screens. Chem Biol 2013; 20: 1352–1363.
Abu Aboud O, Chen C-H, Senapedis W, Baloglu E, Argueta C, Weiss RH . Dual and specific inhibition of NAMPT and PAK4 By KPT-9274 decreases kidney cancer growth. Mol Cancer Ther 2016; 15: 2119–2129.
Fulciniti M, Martinez-Lopez J, Senapedis W, Oliva S, Lakshmi Bandi R, Amodio N et al. Functional role and therapeutic targeting of p21-activated kinase 4 in multiple myeloma. Blood 2017; 129: 2233–2245.
Radu M, Semenova G, Kosoff R, Chernoff J . PAK signalling during the development and progression of cancer. Nat Rev Cancer 2014; 14: 13–25.
Li Y, Shao Y, Tong Y, Shen T, Zhang J, Li Y et al. Nucleo-cytoplasmic shuttling of PAK4 modulates β-catenin intracellular translocation and signaling. Biochim Biophys Acta 2012; 1823: 465–475.
King H, Nicholas NS, Wells CM Chapter Seven - role of p-21-activated kinases in cancer progression. In: KW Jeon (ed). International Review of Cell and Molecular Biology. Academic Press: Elsevier, Amsterdam, The Netherlands. pp 347–387, 2014.
Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ et al. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res 2008; 6: 1215–1224.
Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature 2011; 473: 384–388.
Jiang Y-Y, Lin D-C, Mayakonda A, Hazawa M, Ding L-W, Chien W-W et al. Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut 2016; 66: 1358–1368.
Shames DS, Elkins K, Walter K, Holcomb T, Du P, Mohl D et al. Loss of NAPRT1 expression by tumor-specific promoter methylation provides a novel predictive biomarker for NAMPT inhibitors. Clin Cancer Res 2013; 19: 6912–6923.
Aksoy P, White TA, Thompson M, Chini EN . Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 2006; 345: 1386–1392.
Hu Y, Wang H, Wang Q, Deng H . Overexpression of CD38 decreases cellular NAD levels and alters the expression of proteins involved in energy metabolism and antioxidant defense. J Proteome Res 2014; 13: 786–795.
Aboukameel A, Muqbil I, Senapedis W, Baloglu E, Landesman Y, Shacham S et al. Novel p21-activated kinase 4 (PAK4) allosteric modulators overcome drug resistance and stemness in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2017; 16: 76–87.
Olesen UH, Petersen JG, Garten A, Kiess W, Yoshino J, Imai S-I et al. Target enzyme mutations are the molecular basis for resistance towards pharmacological inhibition of nicotinamide phosphoribosyltransferase. BMC Cancer 2010; 10: 677.
Garten A, Petzold S, Körner A, Imai S-I, Kiess W . Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab 2009; 20: 130–138.
Acknowledgements
We thank Professor Dario Campana (National University of Singapore) for kindly providing OP-1 cells. We thank Professor Chng Wee Joo, Associate Professor Motomi Osato (Cancer Science Institute of Singapore) and Associate Professor Allen Yeoh (National University of Singapore) for sharing materials and advice. We thank Associate Professor Takaomi Sanda, Dr Shojiro Kitajima (Cancer Science Institute of Singapore) and Dr Takahiro Kamiya (National University of Singapore) for helpful discussion. This research was supported by the National Research Foundation Singapore under its Singapore Translational Research (STaR) Investigator Award (NMRC/STaR/0007/2008 and NMRC/STaR/0021/2014), and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), the NMRC Centre Grant awarded to National University Cancer Institute of Singapore, the National Research Foundation Singapore, the Singapore Ministry of Education under its Research Centres of Excellence initiatives and the US National Institutes of Health Grant R01CA026038-35. This study was partially supported by a generous donation from the Melamed family, Reuben Yeroushalmi and Blanche and Steven Koegler.
Author contributions
ST designed the study, performed experiments, analyzed the data and wrote the manuscript. WC performed experiments and analyzed the data. VM and D-CL discussed the data and provided helpful suggestion. AM performed bioinformatics analysis. L-WD, Q-YS, MS, LX, YC, Y-YJ, SG and ML provided helpful suggestion. MM and EP provided PDX B-ALL cells and helpful advice for handling them. WS and EB provided KPT-9274 and its information. HPK supervised and designed the study, discussed the data and helped write the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WS and EB are employees of Karyopharm Therapeutics. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Takao, S., Chien, W., Madan, V. et al. Targeting the vulnerability to NAD+ depletion in B-cell acute lymphoblastic leukemia. Leukemia 32, 616–625 (2018). https://doi.org/10.1038/leu.2017.281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.281
This article is cited by
-
p21-Activated kinases as promising therapeutic targets in hematological malignancies
Leukemia (2022)
-
NAD+ metabolism, stemness, the immune response, and cancer
Signal Transduction and Targeted Therapy (2021)
-
Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) with OT-82 induces DNA damage, cell death, and suppression of tumor growth in preclinical models of Ewing sarcoma
Oncogenesis (2020)
-
Effective targeting of NAMPT in patient-derived xenograft models of high-risk pediatric acute lymphoblastic leukemia
Leukemia (2020)
-
Metabolic gatekeepers to safeguard against autoimmunity and oncogenic B cell transformation
Nature Reviews Immunology (2019)