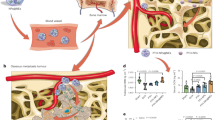
Abstract
The concept of arming antibodies with bioactive payloads for a site-specific therapy of cancer has gained considerable interest in recent years. However, a successful antibody-based targeting approach critically relies on the availability of a tumor-associated target that is not only preferentially expressed in the tumor tissue but is also easily accessible for antibody therapeutics coming from the bloodstream. Here, we perfused the vasculature of healthy and acute myeloid leukemia (AML)-bearing rats with a reactive ester derivative of biotin and subsequently quantified the biotinylated proteins to identify AML-associated bone marrow (BM) antigens accessible from the bloodstream. In total, >1400 proteins were identified. Overall, 181 proteins were >100-fold overexpressed in AML as compared with normal BM. Eleven of the most differentially expressed proteins were further validated by immunohistochemistry and confocal microscopic analyses, including novel antigens highly expressed in AML cells (for example, adaptor-related protein complex 3 β2) and in the leukemia-modified extracellular matrix (ECM) (for example, collagen-VI-α-1). The presented atlas of targetable AML-associated BM proteins provides a valuable basis for the development of monoclonal antibodies that could be used as carriers for a site-specific pharmacodelivery of cytotoxic drugs, cytokines or radionuclides to the BM in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neri D, Bicknell R . Tumour vascular targeting. Nat Rev Cancer 2005; 5: 436–446.
Scott AM, Wolchok JD, Old LJ . Antibody therapy of cancer. Nat Rev Cancer 2012; 12: 278–287.
Schliemann C, Neri D . Antibody-based targeting of the tumor vasculature. Biochim Biophys Acta 2007; 1776: 175–192.
Smaglo BG, Aldeghaither D, Weiner LM . The development of immunoconjugates for targeted cancer therapy. Nat Rev Clin Oncol 2014; 11: 637–648.
Schrama D, Reisfeld RA, Becker JC . Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 2006; 5: 147–159.
Thorpe PE . Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004; 10: 415–427.
Erba PA, Sollini M, Orciuolo E, Traino C, Petrini M, Paganelli G et al. Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J Nucl Med 2012; 53: 922–927.
Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol 2002; 20: 264–269.
Pasche N, Neri D . Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today 2012; 17: 583–590.
Schliemann C, Palumbo A, Zuberbühler K, Villa A, Kaspar M, Trachsel E et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 2009; 113: 2275–2283.
Bernardes GJL, Casi G, Trüssel S, Hartmann I, Schwager K, Scheuermann J et al. A traceless vascular-targeting antibody-drug conjugate for cancer therapy. Angew Chem Int Ed Engl 2012; 51: 941–944.
Perrino E, Steiner M, Krall N, Bernardes GJL, Pretto F, Casi G et al. Curative properties of noninternalizing antibody-drug conjugates based on maytansinoids. Cancer Res 2014; 74: 2569–2578.
Jain RK . Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 1990; 50: 814s–819s.
Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res 2007; 67: 254–261.
Rybak J-N, Rybak J-N, Ettorre A, Kaissling B, Giavazzi R, Neri D et al. In vivo protein biotinylation for identification of organ-specific antigens accessible from the vasculature. Nat Methods 2005; 2: 291–298.
Roesli C, Neri D, Rybak J-N . In vivo protein biotinylation and sample preparation for the proteomic identification of organ- and disease-specific antigens accessible from the vasculature. Nat Protoc 2006; 1: 192–199.
Schliemann C, Roesli C, Kamada H, Fugmann T, Klapper W, Neri D . In vivo biotinylation of the vasculature in B-cell lymphoma identifies BST-2 as a target for antibody-based therapy. Blood 2010; 115: 736–744.
Martens AC, Van Bekkum DW, Hagenbeek A . The BN acute myelocytic leukemia (BNML) (a rat model for studying human acute myelocytic leukemia (AML)). Leukemia 1990; 4: 241–257.
McCormack E, Bruserud Ø, Gjertsen BT . Animal models of acute myelogenous leukaemia - development, application and future perspectives. Leukemia 2005; 19: 687–706.
Roesli C, Fugmann T, Schliemann C, Neri D . A proteomic approach for the identification of vascular markers of liver metastasis. Cancer Res 2010; 70: 309–318.
Hömme C, Krug U, Tidow N, Schulte B, Kühler G, Serve H et al. Low SMC1A protein expression predicts poor survival in acute myeloid leukemia. Oncol Rep 2010; 24: 47–56.
Schliemann C, Bieker R, Padró T, Kessler T, Hintelmann H, Büchner T et al. Expression of angiopoietins and their receptor Tie2 in the bone marrow of patients with acute myeloid leukemia. Haematologica 2006; 91: 1203–1211.
Kreuter M, Woelke K, Bieker R, Schliemann C, Steins M, Buechner T et al. Correlation of neuropilin-1 overexpression to survival in acute myeloid leukemia. Leukemia 2006; 20: 1950–1954.
Uhlen M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419–1260419.
Gattoni-Celli S, Buckner CL, Lazarchick J, Stuart RK, Fernandes DJ . Overexpression of nucleolin in engrafted acute myelogenous leukemia cells. Am J Hematol 2009; 84: 535–538.
Sherman-Baust CA, Weeraratna AT, Rangel LBA, Pizer ES, Cho KR, Schwartz DR et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 2003; 3: 377–386.
Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 2003; 22: 6408–6423.
Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA et al. Adipocyte-derived collagen VI affects early mammary tumor progression In vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest 2005; 115: 1163–1176.
Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M et al. Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem 2008; 283: 10658–10670.
Park J, Scherer PE . Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest 2012; 122: 4243–4256.
Turtoi A, Blomme A, Bianchi E, Maris P, Vannozzi R, Naccarato AG et al. Accessibilome of human glioblastoma: collagen-vi-alpha-1 is a new target and a marker of poor outcome. J Proteome Res 2014; 13: 5660–5669.
Sercu S, Zhang L, Merregaert J . The extracellular matrix protein 1: its molecular interaction and implication in tumor progression. Cancer Invest 2008; 26: 375–384.
Lee K-M, Nam K, Oh S, Lim J, Kim RK, Shim D et al. ECM1 regulates tumor metastasis and CSC-like property through stabilization of β-catenin. Oncogene 2015; 34: 6055–6065.
Han Z, Ni J, Smits P, Underhill CB, Xie B, Chen Y et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. FASEB J 2001; 15: 988–994.
Lal G, Hashimi S, Smith BJ, Lynch CF, Zhang L, Robinson RA et al. Extracellular matrix 1 (ECM1) expression is a novel prognostic marker for poor long-term survival in breast cancer: a Hospital-based Cohort Study in Iowa. Ann Surg Oncol 2009; 16: 2280–2287.
Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G . The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009; 457: 892–895.
Larance M, Lamond AI . Multidimensional proteomics for cell biology. Nat Rev Mol Cell Biol 2015; 16: 269–280.
Aebersold R, Mann M . Mass-spectrometric exploration of proteome structure and function. Nature 2016; 537: 347–355.
Hernandez-Valladares M, Aasebø E, Selheim F, Berven FS, Bruserud Ø . Selecting sample preparation workflows for mass spectrometry-based proteomic and phosphoproteomic analysis of patient samples with acute myeloid leukemia. Proteomes 2016; 4: 1–9.
Eliuk S, Makarov A . Evolution of Orbitrap Mass Spectrometry Instrumentation. Annu Rev Anal Chem (Palo Alto Calif) 2015; 8: 61–80.
Nadler WM, Waidelich D, Kerner A, Hanke S, Berg R, Trumpp A et al. MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. J Proteome Res 2017; 16: 1207–1215.
Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC et al. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell 2009; 16: 281–294.
Cui J-W, Wang J, He K, Jin B-F, Wang H-X, Li W et al. Proteomic analysis of human acute leukemia cells: insight into their classification. Clin Cancer Res 2004; 10: 6887–6896.
Foss EJ, Radulovic D, Stirewalt DL, Radich J, Sala-Torra O, Pogosova-Agadjanyan EL et al. Proteomic classification of acute leukemias by alignment-based quantitation of LC-MS/MS data sets. J Proteome Res 2012; 11: 5005–5010.
Aasebø E, Vaudel M, Mjaavatten O, Gausdal G, Van der Burgh A, Gjertsen BT et al. Performance of super-SILAC based quantitative proteomics for comparison of different acute myeloid leukemia (AML) cell lines. Proteomics 2014; 14: 1971–1976.
Casado P, Rodriguez-Prados J-C, Cosulich SC, Guichard S, Vanhaesebroeck B, Joel S et al. Kinase-substrate enrichment analysis provides insights into the heterogeneity of signaling pathway activation in leukemia cells. Sci Signal 2013; 6: rs6.
Schaab C, Oppermann FS, Klammer M, Pfeifer H, Tebbe A, Oellerich T et al. Global phosphoproteome analysis of human bone marrow reveals predictive phosphorylation markers for the treatment of acute myeloid leukemia with quizartinib. Leukemia 2014; 28: 716–719.
Roolf C, Dybowski N, Sekora A, Mueller S, Knuebel G, Tebbe A et al. Phosphoproteome analysis reveals differential mode of action of sorafenib in wildtype and mutated FLT3 AML cells. Mol Cell Proteomics 2017; 16: 1365–1376, mcp.M117.067462.
Strassberger V, Gutbrodt KL, Krall N, Roesli C, Takizawa H, Manz MG et al. A comprehensive surface proteome analysis of myeloid leukemia cell lines for therapeutic antibody development. J Proteomics 2014; 99: 138–151.
Bonardi F, Fusetti F, Deelen P, van Gosliga D, Vellenga E, Schuringa JJ . A proteomics and transcriptomics approach to identify leukemic stem cell (LSC) markers. Mol Cell Proteomics 2013; 12: 626–637.
Schliemann C, Bieker R, Thoennissen N, Gerss J, Liersch R, Kessler T et al. Circulating angiopoietin-2 is a strong prognostic factor in acute myeloid leukemia. Leukemia 2007; 21: 1901–1906.
Schlöndorff J, Blobel CP . Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci 1999; 112 (Pt 21): 3603–3617.
Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003; 362: 869–875.
McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM . Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res 2004; 10: 314–323.
Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 2005; 168: 633–642.
Poppe B, Vandesompele J, Schoch C, Lindvall C, Mrozek K, Bloomfield CD et al. Expression analyses identify MLL as a prominent target of 11q23 amplification and support an etiologic role for MLL gain of function in myeloid malignancies. Blood 2004; 103: 229–235.
Cogle CR, Goldman DC, Madlambayan GJ, Leon RP, Masri AlA, Clark HA et al. Functional integration of acute myeloid leukemia into the vascular niche. Leukemia 2014; 28: 1978–1987.
Newman LS, McKeever MO, Okano HJ, Darnell RB . Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 1995; 82: 773–783.
Newell-Litwa K, Salazar G, Smith Y, Faundez V . Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell 2009; 20: 1441–1453.
Sanuki R, Watanabe S, Sugita Y, Irie S, Kozuka T, Shimada M et al. Protein-4.1G-mediated membrane trafficking is essential for correct rod synaptic location in the retina and for normal visual function. Cell Rep 2015; 10: 796–808.
Hanke SA, Kerner A, Nadler WM, Trumpp A, Zhang Y, Rösli CP . The pivotal role of reactivity in the design of novel biotinylation reagents for the chemical-proteomics-based identification of vascular accessible biomarkers. J Proteomics 2016; 141: 57–66.
Epstein AL, Chen FM, Taylor CR . A novel method for the detection of necrotic lesions in human cancers. Cancer Res 1988; 48: 5842–5848.
Chen S, Yu L, Jiang C, Zhao Y, Sun D, Li S et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol 2005; 23: 1538–1547.
Begent RH . The value of carcinoembryonic antigen measurement in clinical practice. Ann Clin Biochem 1984; 21 (Pt 4): 231–238.
Gasiorowski RE, Clark GJ, Bradstock K, Hart DNJ . Antibody therapy for acute myeloid leukaemia. Br J Haematol 2014; 164: 481–495.
Busfield SJ, Biondo M, Wong M, Ramshaw HS, Lee EM, Ghosh S et al. Targeting of acute myeloid leukemia in vitro and In vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia 2014; 28: 2213–2221.
Hofmann M, Große-Hovest L, Nübling T, Pyż E, Bamberg ML, Aulwurm S et al. Generation, selection and preclinical characterization of an Fc-optimized FLT3 antibody for the treatment of myeloid leukemia. Leukemia 2012; 26: 1228–1237.
Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med 2013; 5: 201ra118.
Schliemann C, Gutbrodt KL, Kerkhoff A, Pohlen M, Wiebe S, Silling G et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res 2015; 3: 547–556.
Eksioglu EA, Chen X, Heider KH, Rueter B, McGraw KL, Basiorka AA et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Nat Med 2017; 96: 483.
Acknowledgements
This study is dedicated to the memory of Professor Thomas Büchner. We thank Irina Arnhold, Heike Hintelmann, Verena Mantke, Petra Fischer and Cordula Westermann for their technical assistance. This work was supported by the fund ‘Innovative Medical Research’ of the University of Münster Medical School (Grants SC211008 (to C Schliemann and SR), SC111411 (to C Schliemann) and SC221410 (to LA and C Schliemann)). WEB and GL are supported by the Deutsche Forschungsgemeinschaft (DFG EXC 1003, Cluster of excellence ‘Cells in Motion’).
Author contributions
LA, SR, CR and C Schliemann designed the project, performed the majority of the experiments, analyzed results and wrote the manuscript; ASB and CR performed the proteomic analysis; C Schwöppe, TK, LHS, TS, CB and J-HM contributed to immunohistochemistry experiments; ACM provided the BNML cell line; SR, DK and LA performed the perfusion experiments; WEB, WH and EW provided clinical data and human AML BM specimens; FN and JH performed confocal microscopy analyses; CM-T, GL, RMM, DN and WEB provided discussion and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Angenendt, L., Reuter, S., Kentrup, D. et al. An atlas of bloodstream-accessible bone marrow proteins for site-directed therapy of acute myeloid leukemia. Leukemia 32, 510–519 (2018). https://doi.org/10.1038/leu.2017.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.208
This article is cited by
-
Adrenomedullin-CALCRL axis controls relapse-initiating drug tolerant acute myeloid leukemia cells
Nature Communications (2021)
-
A mass spectrometry guided approach for the identification of novel vaccine candidates in gram-negative pathogens
Scientific Reports (2019)
-
Stromal collagen type VI associates with features of malignancy and predicts poor prognosis in salivary gland cancer
Cellular Oncology (2018)