Abstract
Autologous stem cell transplantation (ASCT) is a standard treatment for eligible multiple myeloma (MM) patients, but many patients will relapse after ASCT and require subsequent therapy. The proteasome inhibitor carfilzomib is approved for relapsed or refractory MM (RRMM). In phase 3 trials, carfilzomib-based regimens (ASPIRE, carfilzomib–lenalidomide–dexamethasone; ENDEAVOR, carfilzomib–dexamethasone) demonstrated superior progression-free survival (PFS) compared with standard therapies for RRMM (ASPIRE: lenalidomide–dexamethasone; ENDEAVOR, bortezomib–dexamethasone). This subgroup analysis of ASPIRE and ENDEAVOR evaluated outcomes according to prior ASCT status. In total, 446 patients in ASPIRE and 538 in ENDEAVOR had prior ASCT. Median PFS was longer for carfilzomib-based regimens vs non-carfilzomib-based regimens for patients with prior ASCT (ASPIRE: 26.3 vs 17.8 months (hazard ratio (HR)=0.68); ENDEAVOR: not estimable vs 10.2 months (HR=0.61)), those with one prior line of therapy that included ASCT (ASPIRE: 29.7 vs 17.8 months (HR=0.70); ENDEAVOR: not estimable vs 11.2 months (HR=0.46)), and those without prior ASCT (ASPIRE: 26.4 vs 16.6 months (HR=0.76); ENDEAVOR: 17.7 vs 8.5 months (HR=0.43)). Overall response rates also favored the carfilzomib-based regimens. No new safety signals were detected. This analysis suggests that carfilzomib-based treatment may lead to improvement in PFS and response rates regardless of prior transplant status. Further evaluation is warranted.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is an aggressive and invariably fatal malignancy of plasma cell origin, with an estimated 120 000 new cases occurring annually worldwide, an incidence rate that is expected to rise along with the aging world population.1 Autologous stem cell transplantation (ASCT) is a standard of care for newly diagnosed MM patients who have no prohibitive comorbidities. Studies have demonstrated that ASCT upfront after induction regimens, including novel agents, significantly improves progression-free survival (PFS) compared with these regimens alone.2 Administration of the immunomodulatory drugs thalidomide and lenalidomide and the proteasome inhibitor bortezomib both before and after ASCT has improved response rates and increased survival rates for MM patients. Modern combination therapies such as carfilzomib, lenalidomide and dexamethasone (KRd) have demonstrated high rates of complete response and minimal residual disease negativity, especially when combined with ASCT,3, 4 but not all patients achieved these deep remissions. Despite the different therapeutic strategies, relapse occurs in a majority of patients due to residual disease, and duration of response is generally shorter with each relapse over the course of the disease. Treatment options such as repeat ASCT with induction regimens including bortezomib or immunomodulatory drugs are used for the treatment of relapsed/refractory multiple myeloma (RRMM).5, 6 However, ASCT at the time of relapse is not recommended if the time to relapse after first ASCT is <12 months.7, 8, 9, 10
Bortezomib plus dexamethasone and lenalidomide plus dexamethasone are considered standard of care regimens for MM patients who have relapsed after one prior line of therapy.11, 12, 13, 14
Carfilzomib is a second-generation, irreversible, epoxyketone proteasome inhibitor approved in the United States as a single agent for the treatment of RRMM and also in the United States and European Union as combination therapy with dexamethasone or lenalidomide plus dexamethasone for RRMM.15, 16 The approval of these carfilzomib-based combination regimens was based on interim results from the phase 3 randomized ASPIRE and ENDEAVOR studies. The ASPIRE study showed superior PFS and disease response with KRd vs Rd in patients with RRMM.17 Similarly, the ENDEAVOR study demonstrated superiority of carfilzomib plus dexamethasone (Kd) vs bortezomib plus dexamethasone (Vd) in patients with RRMM.18 Both ASPIRE and ENDEAVOR were conducted in relapsed or refractory patients after 1–3 prior lines of therapy. Based on results for carfilzomib in the ASPIRE and ENDEAVOR studies, we hypothesized that carfilzomib-based regimens would provide superior clinical outcomes vs Rd and Vd, regardless of prior ASCT status. This subgroup analysis evaluated the efficacy and safety of carfilzomib-based regimens in the ASPIRE and ENDEAVOR studies according to prior exposure to ASCT in any prior line of therapy, prior exposure to first-line ASCT and time to relapse after first-line ASCT.
Patients and methods
The ASPIRE and ENDEAVOR studies have been described in detail in previously published reports.17, 18 To summarize, ASPIRE (NCT01080391) and ENDEAVOR (NCT01568866) were both randomized, open-label, phase 3 studies. Adult patients (age⩾18 years) with RRMM and measurable disease who had received 1–3 prior lines of therapy were eligible to enroll in the studies.
In the ASPIRE study, prior bortezomib exposure was allowed, provided that patients had no disease progression during treatment with bortezomib. Patients who were previously treated with Rd were also eligible to participate in the ASPIRE study, provided that they had not experienced adverse events (AEs) that led to treatment discontinuation, disease progression during the first 3 months of treatment or disease progression at any time if Rd was their most recent treatment. In the ENDEAVOR study, patients previously treated with bortezomib or carfilzomib were eligible provided they had achieved at least a partial response to treatment, had at least a 6-month interval without bortezomib or carfilzomib treatment prior to enrollment and did not discontinue bortezomib or carfilzomib as a result of toxicity. All patients enrolled in the ASPIRE and ENDEAVOR studies were required to have adequate hepatic, hematological and renal function. Patients were excluded from these studies if they had New York Heart Association class III or IV heart failure, myocardial infarction within 4 months prior to randomization or grade ⩾3 peripheral neuropathy within 14 days prior to randomization. In addition, patients in ENDEAVOR were required to have a left ventricular ejection fraction of ⩾40%. All patients in both studies provided written informed consent. The study protocol received institutional review board or ethics committee approval by all participating institutions.
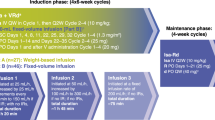
In the ASPIRE study, patients were randomized 1:1 to receive KRd or Rd in 28-day cycles until disease progression, withdrawal of consent or development of toxic effects. The stratification factors used for randomization were β2-microglobulin level, prior bortezomib therapy and prior lenalidomide therapy. Within each stratum, patients were randomized using a blocked randomization scheme. Patients received carfilzomib as a 10-min intravenous infusion on days 1, 2, 8, 9, 15 and 16 (20 mg/m2 on days 1 and 2 of cycle 1; 27 mg/m2 thereafter) during cycles 1–12. Carfilzomib was omitted on days 8 and 9 of cycles 13–18 and was discontinued after 18 cycles. All patients received lenalidomide (25 mg) on days 1–21 and dexamethasone (40 mg) on days 1, 8, 15 and 22.
Patients in the ENDEAVOR study were randomly assigned 1:1 to receive Kd or Vd. The stratification factors used for randomization were prior proteasome inhibitor therapy, prior lines of treatment, International Staging System stage for MM prognosis and planned route of bortezomib administration (for those randomized to the Vd arm). Similar to the ASPIRE study, patients were randomized using a blocked randomization scheme. Patients in the Kd arm received carfilzomib (30- min intravenous infusion) on days 1, 2, 8, 9, 15 and 16 (20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter) and dexamethasone (20 mg) on days 1, 2, 8, 9, 15, 16, 22 and 23 of a 28- day cycle until disease progression, withdrawal of consent or development of toxic effects. Patients in the Vd arm received bortezomib (1.3 mg/m2) on days 1, 4, 8 and 11 and dexamethasone (20 mg) on days 1, 2, 4, 5, 8, 9, 11 and 12 of a 21-day cycle.
In this subgroup analysis, patients were assigned to a subgroup based on transplant status (Supplementary Figure S1): (1) all patients who had previously undergone ASCT (ASCT) and (2) patients who had not previously undergone ASCT (no ASCT). A further subgroup was considered of patients who had undergone ASCT specifically as first-line treatment and then enrolled in ASPIRE or ENDEAVOR immediately upon first relapse (1R1T). The 1R1T group was further subdivided by time to relapse (<12 months (early relapsers) or ⩾12 months (late relapsers)). The intent-to-treat population was used for the efficacy analysis. Patients who received ⩾1 dose of study treatment were used for safety analysis. The analyses of ASPIRE and ENDEAVOR patients were conducted separately.
The primary outcome was PFS, which was defined as the time from randomization until disease progression or death due to any cause. Secondary end points included overall response rate (ORR) and safety. ORR was defined as the proportion of patients achieving a best response of the following: partial response, very good partial response, complete response, or stringent complete response. Treatment responses and disease progression were assessed centrally by an independent review committee that was blinded to the treatment arm.
Kaplan–Meier method was used to summarize PFS. The hazard ratios (HRs) associated with PFS were estimated via Cox regression models. The non-parametric log-rank test with no assumptions was used to compare data between treatment groups.
Results
Treatment and baseline characteristics
The cutoff dates for the prespecified interim analyses were June 16, 2014 for the ASPIRE study and November 10, 2014 for the ENDEAVOR study. The ASCT subgroups included in this subgroup analysis are shown in Supplementary Figure S1. In ASPIRE, 346 patients had no prior ASCT (KRd, n=179; Rd, n=167) and 446 had prior ASCT (KRd, n=217; Rd, n=229). In ENDEAVOR, 391 patients did not have prior ASCT (Kd, n=198; Vd, n=193) and 538 had prior ASCT (Kd, n=266; Vd, n=272). Of those with prior ASCT, 430 patients enrolled in ASPIRE (n=166) or ENDEAVOR (n=264) upon first relapse (1R1T group). Within the 1R1T subgroup, 89 patients were early relapsers (ASPIRE, n=38; ENDEAVOR, n=51) and 337 were late relapsers (ASPIRE, n=128; ENDEAVOR, n=209).
Patient demographics and baseline characteristics in the ASPIRE and ENDEAVOR studies were generally balanced between treatment arms within the subgroups based on prior ASCT exposure (Table 1 and Supplementary Table S1). Patients with prior ASCT were younger and less likely to have renal dysfunction and International Staging System stage II–III disease at baseline than patients with no prior ASCT.
PFS for ASPIRE and ENDEAVOR prior ASCT groups
Median PFS was longer for the carfilzomib-based regimens than for the Rd and Vd arms for all ASCT subgroups (Table 2; Figures 1 and 2). In the prior ASCT group, median PFS was 26.3 vs 17.8 months for KRd vs Rd (HR, 0.68; 95% confidence interval (CI), 0.53–0.87) and not reached vs 10.2 months for Kd vs Vd (HR, 0.61; 95% CI, 0.47–0.79). In the 1R1T group, median PFS was 29.7 vs 17.8 months for KRd vs Rd (HR, 0.70; 95% CI, 0.46–1.07) and not reached vs 11.2 months for Kd vs Vd (HR, 0.46; 95% CI, 0.30–0.69). In the no prior ASCT group, median PFS was 26.4 vs 16.6 months for KRd vs Rd (HR, 0.76; 95% CI, 0.57–1.01) and 17.7 vs 8.5 months for Kd vs Vd (HR, 0.43; 95% CI, 0.32–0.59). Median PFS was longer with carfilzomib-based regimens compared with non-carfilzomib-based regimens for both early and late relapsers within the 1R1T subgroup (Table 3; Figures 3 and 4). The 1- and 2-year PFS rates were higher in the KRd arm vs the Rd arm in the prior and no prior ASCT subgroups, as well as in the 1R1T subgroup. PFS rates were also higher in the Kd arm vs the Vd arm in all transplant subgroups (Table 2).
Kaplan–Meier curves for PFS by time to relapse in the ASPIRE trial (1R1T group): (a) early relapsers and (b) late relapsers. Vertical dashed line indicates the time point after which carfilzomib was discontinued according to study protocol. Early relapsers refers to patients relapsing <12 months post-ASCT. Late relapsers refers to patients relapsing ⩾12 months post-ASCT.
Response rates for ASPIRE and ENDEAVOR prior ASCT groups
In the ASPIRE study, the ORRs were markedly higher for the KRd arms than in the Rd arms in the no prior, prior and 1R1T subgroups, including patients with early and late relapse (Tables 2 and 3). A similar trend was reported for Kd vs Vd in the ENDEAVOR trial. The proportion of patients achieving complete response or better was higher for the carfilzomib-based regimens across all subgroups in both studies (Tables 2 and 3).
Safety
Table 4 provides a summary of AEs. All grade and grade ⩾3 anemia were reported more frequently in carfilzomib-treated patients vs non-carfilzomib-treated patients in the prior ASCT subgroup of ASPIRE and ENDEAVOR. The proportion of patients with grade ⩾3 thrombocytopenia was higher in the carfilzomib vs non-carfilzomib arms in the prior ASCT subgroup of ASPIRE. In addition, the incidences of all-grade and grade⩾3 hypertension were greater in carfilzomib-treated patients than in non-carfilzomib-treated patients in all subgroups of both studies.
All grade peripheral neuropathy occurred more frequently in the Vd arm than in the Kd arm in the prior ASCT and no ASCT subgroups of the ENDEAVOR study. In ASPIRE, there was no difference in the rate of all-grade peripheral neuropathy between the prior ASCT and no ASCT treatment arms. The frequencies of all-grade and grade ⩾3 cardiac failure were higher for Kd-treated patients compared with Vd-treated patients in the prior ASCT and no ASCT subgroups of the ENDEAVOR study. The frequencies of all-grade and grade ⩾3 cardiac failure were higher for KRd-treated patients compared with Rd-treated patients in the no ASCT subgroup. All grade and grade ⩾3 acute renal failure occurred more frequently in the KRd arm than in the Rd arm and in the Kd arm than in the Vd arm in the prior ASCT group.
Discussion
ASCT is considered a standard of care for patients with MM who are eligible for the procedure. Nevertheless, the vast majority of patients will relapse or experience disease progression and eventually require subsequent treatment. The efficacy of novel combination treatments in patients with MM based on ASCT status is relevant because ASCT may potentially influence the efficacy of therapy. ASCT in young, newly diagnosed MM patients is a complementary treatment strategy rather than an alternative one, even after induction with novel agents that result in median PFS of up to 60 months.19, 20, 21 Despite improvements in survival following ASCT, residual clonal malignant plasma cells may escape the effects of therapy and survive.22, 23 Expression of certain markers on plasma cells such as CD45, CD46 and CD56 could predict a higher risk of relapse following ASCT.24, 25, 26, 27, 28 In addition, the presence of high-risk cytogenetic abnormalities is associated with shorter duration of responses, and the frequency of specific genetic aberrations such as deletion t(4:14) is higher in younger, transplant-eligible patients than elderly patients.29 High risk transplant-eligible patients have a different course of disease and represent a group with an unmet medical need. Examining therapies for posttransplant relapse is important.
In the randomized phase 3 ASPIRE and ENDEAVOR studies, patients with RRMM having undergone 1–3 prior lines of therapy demonstrated significantly improved PFS with KRd vs Rd and with Kd vs Vd, respectively.17, 18 This subgroup analysis was conducted on the ASPIRE and ENDEAVOR data to compare treatments by prior ASCT exposure, including a subgroup of patients enrolled at first-line ASCT relapse, and further by time to relapse after first-line ASCT. Within ASPIRE and ENDEAVOR, the increase in PFS and longer response rates observed with carfilzomib-based regimens vs non-carfilzomib-based regimens were consistent across ASCT subgroups. Notably, patients with prior ASCT who were treated with KRd or Kd had 2-year PFS rates >50%, which is remarkable for treatment regimens not including repeat ASCT after post-ASCT relapse. Similar patients in the non-carfilzomib arms did not achieve such PFS rates.
The ORR and the proportion of patients achieving complete response or better were also greater for the carfilzomib-based regimens in the prior ASCT, 1R1T and no ASCT groups. Approximately three times as many KRd patients as Rd patients, and twice as many Kd patients as Vd patients, achieved complete response or better in all subgroups defined by prior ASCT status. The fact that a higher proportion of carfilzomib-treated patients achieved complete response or better in all transplant subgroups is encouraging, given that previous studies have indicated an association between depth of response and improved survival in patients with MM.30 Collectively, the results obtained from these two phase 3 studies suggest that, irrespective of prior transplant status, carfilzomib-based regimens may provide increased clinical benefit over Rd and Vd for patients with RRMM. Additional robustly powered studies are required to confirm that carfilzomib-based treatments provide increased clinical efficacy over Rd and Vd.
Within the 1R1T subgroup, comprising patients enrolled in first relapse after a frontline ASCT, late relapsers (⩾12 months) generally fared better than early relapsers (<12 months), which is not surprising given that patients who relapse within 1 year of ASCT have more aggressive disease and poorer outcomes.31, 32 Notably, there was a consistency of benefit for the carfilzomib-based regimens for the early and late relapse subgroups. Patients who progressed within 1 year after ASCT had a median PFS of 15.2 months with KRd treatment, which is longer than the remission period after first-line ASCT. Patients with longer remission after ASCT (⩾12 months) had a median PFS approaching 3 years with KRd treatment despite reduction in carfilzomib dose intensity after 12 cycles and discontinuation of carfilzomib at 18 cycles.
With respect to survival outcomes, PFS HRs favored the carfilzomib-based regimens for both early relapsers (KRd vs Rd, 0.75; Kd vs Vd, 0.36) and late relapsers (KRd vs Rd, 0.72; Kd vs Vd, 0.48).
In this post hoc analysis of prior transplant subgroups of ASPIRE and ENDEAVOR, we observed higher rates of grade ⩾3 AEs in patients receiving carfilzomib-based regimens vs the non-carfilzomib regimens. The frequency of AE-related treatment discontinuation or death was similar between the KRd and Rd arms in all subgroups of the ASPIRE study. In the ENDEAVOR study, the frequency of AE-related treatment discontinuation or death was similar between the Kd and Vd arms in the prior ASCT and no ASCT subgroups. Although any-grade hypertension occurred ⩾5% more frequently with KRd vs Rd and with Kd vs Vd in all subgroups, hypertension is a recognized and manageable complication of carfilzomib. Cardiac failure AEs were more common in the no ASCT group than in the prior ASCT group in all treatment arms, which is expected based on baseline characteristics. In the prior ASCT subgroups of both studies, acute renal failure AEs were more frequently reported in patients receiving carfilzomib-based regimens than non-carfilzomib regimens. These safety findings were generally similar to those observed in the primary analyses of the ASPIRE and ENDEAVOR populations.
The proportions of patients in ASPIRE and ENDEAVOR who had undergone stem cell transplant prior to enrollment (56.3% and 57.9%, respectively) were similar to those in other trials comparing novel combination regimens to standards of care.33, 34
For patients relapsing after prior treatment with or without ASCT, either of these two carfilzomib-based regimens may be implemented as subsequent therapy, as they potentially offer longer PFS than other currently available regimens. Additionally, carfilzomib is a proteasome inhibitor that leads to longer PFS in RRMM patients than bortezomib (bortezomib as retreatment or in bortezomib-naive patients). The majority of patients receiving induction therapy followed by ASCT are exposed to bortezomib in first line, making these results particularly relevant. KRd and Kd show improved clinical outcomes for patients who have relapsed following ASCT, including patients at first relapse. KRd and Kd also show superior clinical efficacy in patients with RRMM with no prior ASCT who are generally older and potentially less fit.
There is evidence that treating patients with RRMM early in the course of their disease, particularly at first relapse, provides better outcomes than subsequent therapy administered later. For example, in a study by Stadtmauer et al.,35 Rd prolonged PFS and increased ORR in patients with RRMM at first relapse vs patients offered later subsequent treatment. In the ASPIRE study, the use of KRd after first relapse improved median PFS by 12 months (HR, KRd vs Rd; 0.694) compared with 9 months (HR, KRd vs Rd; 0.688) in patients with⩾2 prior lines of therapy.36 Similarly, in the ENDEAVOR study, median PFS (Kd vs Vd) was longer for patients with one prior line of therapy than in patients with ⩾2 prior lines of therapy.37
To date, no head to head study has been performed to directly compare the efficacy between KRd and Kd in RRMM. Nevertheless, triplet combinations are known to be superior in efficacy to doublets. In this study, KRd delivered 29.7 months of PFS in patients who progressed following ASCT and subsequently enrolled in ASPIRE at first relapse, and may therefore be considered an appropriate treatment choice. However, if patients have progressed or are intolerant to lenalidomide treatment, then Kd would be an appropriate immunomodulatory drug-free treatment regimen.
Aside from Rd and KRd, there are other Rd-based regimens, including elotuzumab–Rd, daratumumab–Rd and ixazomib–Rd, that have been evaluated for their efficacy in treating RRMM. However, there are no prospective studies that compare these newer three-agent combinations. The appropriate treatment regimen upon relapse after ASCT should be individualized and based on disease and patient characteristics, including patient’s age, comorbidities, the number and type of prior regimens, the risk of developing toxicity and the aggressiveness of the disease.
Although this analysis demonstrates the relative efficacy and safety of carfilzomib-based regimens over Rd and Vd in a population of patients with and without prior ASCT, its design has some potential limitations. One is its open-label design, which could introduce bias. Additionally, this was a post hoc analysis with no preplanned hypothesis testing. Another limitation is the relatively small number of patients who received lenalidomide maintenance following ASCT in the ASPIRE and ENDEAVOR trials. A number of studies have demonstrated a PFS benefit for lenalidomide maintenance in the posttransplant setting.38, 39, 40 and a majority of US-based ASCT recipients receive lenalidomide maintenance following ASCT. Evidence, including a recent meta-analysis by Attal et al.,41 has shown that overall survival is significantly prolonged in patients receiving lenalidomide maintenance after ASCT. The present analysis would have been further strengthened if information on post-ASCT lenalidomide maintenance in ASPIRE and ENDEAVOR were robust enough for analysis.
In conclusion, this post hoc analysis suggests that carfilzomib-based treatment leads to clinically meaningful improvements in PFS and ORR compared with Rd and Vd in patients with and without prior ASCT, including those in 1R1T. These results strengthen the hypothesis that KRd and Rd have favorable benefit-risk profiles in patients with RRMM, irrespective of ASCT status or time to 1R1T.
References
Ludwig H, Miguel JS, Dimopoulos MA, Palumbo A, Garcia Sanz R, Powles R et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia 2014; 28: 981–992.
Attal M, Lauwers-Cances V, Hulin C, Facon T, Caillot D, Escoffre M et al. Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial). Blood 2015; 125: 391 (abstract).
Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol 2015; 1: 746–754.
Zimmerman TM, Griffith KA, Jasielec J, Rosenbaum CA, McDonnell K, Waite-Marin J et al. Phase II MMRC trial of extended treatment with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (DEX) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM). J Clin Oncol 2015; 33: Abstract 8510.
Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant 2013; 19: 760–766.
Sellner L, Heiss C, Benner A, Raab MS, Hillengass J, Hose D et al. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer 2013; 119: 2438–2446.
Holstein SA, Richardson PG, Laubach JP, McCarthy PL . Management of relapsed multiple myeloma after autologous stem cell transplantation. Biol Blood Marrow Transplant 2015; 21: 793–798.
Nooka AK, Kastritis E, Dimopoulos MA, Lonial S . Treatment options for relapsed and refractory multiply myeloma. Blood 2015; 125: 3085–3099.
Lemieux E, Hulin C, Caillot D, Tardy S, Dorvaux V, Michel J et al. Autologous stem cell transplantation: an effective salvage therapy in multiple myeloma. Biol Blood Marrow Transplant 2013; 19: 445–449.
Olin R, Vogl D, Porter D, Luger SM, Schuster SJ, Tsai DE et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant 2009; 43: 417–422.
NCCN Multiple Myeloma. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) version 3 2016. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
EMEA Velcade: Product Information. EMEA: London, UK, 2008.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 2007; 357: 2133–2142.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007; 357: 2123–2132.
US FDA Kyprolis: Product Information. US FDA: Maryland, USA, 2016.
EMEA Kyprolis: Product Information. EMEA: London, UK, 2016.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 373: 142–152.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 2016; 17: 27–38.
Rosinol L, Kumar S, Moreau P, Cavo M . Initial treatment of transplant-eligible patients in multiple myeloma. Expert Rev Hematol 2014; 7: 43–53.
Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Blade J et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 2011; 117: 6063–6073.
Attal M, Harousseau JL . Role of autologous stem-cell transplantation in multiple myeloma. Recent Results Cancer Res 2011; 183: 189–206.
Feyler S, Selby PJ, Cook G . Regulating the regulators in cancer immunosuppression in multiple myeloma (MM). Blood Rev 2013; 27: 155–164.
Ribatti D, Moschetta M, Vacca A . Microenvironment and multiple myeloma spread. Thromb Res 2014; 133: S102–S106.
Pellat-Deceunynck C, Battaile R . Normal and malignant human plasma cells: proliferation, differentiation, and expansions in relation to CD45 expression. Blood Cells Mol Dis 2004; 32: 293–301.
Asosingh K, De Raeve H, Van Riet I, Van Camp B, Vanderkerken K . Multiple myeloma tumor progression in the 5T2MM murine model is a multistage and dynamic process of differentiation, proliferation, invasion, and apoptosis. Blood 2003; 101: 3136–3141.
Kraj M, Sokolowska U, Kopec-Szlezak J, Poglod R, Kruk B, Wozniak J et al. Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leuk Lymphoma 2008; 49: 298–305.
Hanamura I, Stewart J, Huang Y, Zhan F, Santra M, Sawyer JR et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases in MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 2006; 108: 1724–1732.
Moreau P, Robillard N, Avet-Loiseau H, Pineau D, Morineau N, Milipied N et al. Patients with CD45 negative multiple myeloma receiving high-dose therapy have a shorter survival than those with CD45 positive multiple myeloma. Haematologica 2004; 89: 547–551.
Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myelome experience. J Clin Oncol 2013; 31: 2806–2809.
Lonial S, Anderson KC . Association of response endpoints with survival outcomes in multiple myeloma. Leukemia 2014; 28: 258–268.
Majithia N, Rajkumar SV, Lacy MQ, Buadi A, Dispenzieri M, Hayman SR et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia 2016; 30: 2208–2213.
Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V . Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents. Bone Marrow Transplant 2015; 50: 2015–2018.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 374: 1621–1634.
Stadtmauer EA, Weber DM, Niesvizky R, Belch A, Prince MH, San Miguel JF et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol 2009; 82: 426–432.
Dimopoulos MA, Stewart KA, Rajkumar SV, Masszi T, Oriol A, Hajek R et al. Effect of carfilzomib, lenalidomide, and dexamethasone (KRd) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma (RMM) by line of therapy: secondary analysis from an interim analysis of the phase III study ASPIRE (NCT01080391). J Clin Oncol 2015; 33 (suppl): Abstract 8525.
Moreau P, Joshua D, Chng WJ, Palumbo A, Goldschmidt H, Hajek R et al. Impact of prior treatment on patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone vs bortezomib and dexamethasone in the phase 3 ENDEAVOR study. Leukemia 2016; 19: 1782–1791.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781.
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366: 1759–1769.
Attal M, Palumbo A, Holstein SA, Lauwers-Cances V, Petrucci MT, Richardson PG et al. Lenalidomide (LEN) maintenance (MNTC) after high-dose melphalan and autologous stem cell transplant (ASCT) in multiple myeloma (MM): a meta-analysis (MA) of overall survival (OS). J Clin Oncol 2016; 34 (suppl): Abstract 8001.
Acknowledgements
The ASPIRE and ENDEAVOR studies were supported by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. We thank Jacqueline Sayyah, PhD, of Amgen Inc. for medical writing support and BlueMomentum, an Ashfield company, for editorial and medical writing assistance.
Author contributions
PH, MVM, RA, SK, WB, HL, KS, RH, PM, DSS and HG designed the work, acquired data, had an important role in interpreting results, drafted the manuscript and approved the final version. SKA and KI conceived and designed the work, had an important role in interpreting results, drafted the manuscript and approved the final version. SF and MO acquired data, had an important role in interpreting the results, drafted the manuscript and approved the final version. The corresponding author confirms that she has had full access to the data in the study and final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MVM has received personal fees from Janssen, Celgene, BMS, Amgen and Takeda. PH has received grants and personal fees from Amgen, Celgene, Takeda, Spectrum and BMS. RA has no relationships to disclose. SK has received personal fees from Amgen, Celgene, Bristol-Myers Squibb and Onyx. WB has received grants and personal fees from Amgen and Celgene. HL has received grants from Takeda, Amgen and Janssen and personal fees from Takeda, Celgene, Amgen and Bristol-Myers Squibb. KS has received personal fees from Amgen, Celgene, Janssen and Takeda. RH has served as a consultant for and received honoraria from Janssen, Celgene and Amgen. PM has served as a consultant for and received honoraria from Celgene, Onyx, Janssen, Novartis and Millennium. DSS has served on the speakers’ bureau for and received honoraria from Celgene, Millennium and Onyx. SF, MO, SKA and KI are employees at Amgen. HG has served as a consultant for Amgen, Celgene, Janssen, Takeda, Novartis, Onyx and BMS and has received research funding and honoraria from Janssen, Celgene, Novartis, Chugai and Millennium.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hari, P., Mateos, MV., Abonour, R. et al. Efficacy and safety of carfilzomib regimens in multiple myeloma patients relapsing after autologous stem cell transplant: ASPIRE and ENDEAVOR outcomes. Leukemia 31, 2630–2641 (2017). https://doi.org/10.1038/leu.2017.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.122
This article is cited by
-
Copy number evolution and its relationship with patient outcome—an analysis of 178 matched presentation-relapse tumor pairs from the Myeloma XI trial
Leukemia (2021)
-
A phase 2 study of carfilzomib, cyclophosphamide and dexamethasone as frontline treatment for transplant-eligible MM with high-risk features (SGH-MM1)
Blood Cancer Journal (2021)
-
Carfilzomib, lenalidomide, and dexamethasone in relapsed/refractory multiple myeloma patients: the real-life experience of Rete Ematologica Pugliese (REP)
Annals of Hematology (2021)
-
Emerging agents and regimens for multiple myeloma
Journal of Hematology & Oncology (2020)
-
Improved outcome in patients following autologous stem cell transplantation for multiple myeloma in south eastern Norway 2001–2010: a retrospective, population based analysis
BMC Cancer (2018)