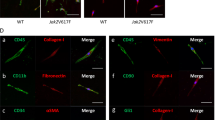
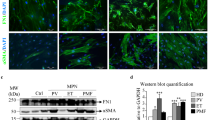
Abstract
Myelofibrosis (MF) may be caused by various pathogenic mechanisms such as elevation in circulating cytokine levels, cellular interactions and genetic mutations. However, the underlying mechanism of MF still remains unknown. Recent studies have revealed that fibrocytes, the spindle-shaped fibroblast-like hematopoietic cells, and the thrombopoietin (TPO)/myeloproliferative leukemia protein (MPL; TPO receptor) signaling pathway play a certain role in the development of MF. In the present study, we aimed to investigate the relationship between fibrocytes and MPL activation. We showed that TPO or a TPO receptor agonist directly induces fibrocyte differentiation using murine fibrocyte cell lines and a murine MF model. Conversely, elimination of macrophages expressing MPL by clodronate liposomes reversed the MF phenotype of the murine model, suggesting that fibrocyte differentiation induced by MPL activation contributes to the progression of MF. Furthermore, we revealed that SLAMF7high MPLhigh monocytes in human peripheral blood mononuclear cells were possible fibrocyte precursors and that these cells increased in number in MF patients not treated with ruxolitinib. Our findings confirmed a link between fibrocytes and the TPO/MPL signaling pathway, which could result in a greater understanding of the pathogenesis of MF and lead to the development of novel therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
19 September 2018
Owing to the insufficient specificity of the anti-myeloproliferative leukemia protein (MPL) antibody in the original version of this Article, Figure 6 and parts of Figures 2a, 4e, and 5a do not represent the correct information. The corrected version of Figure 6 is shown below and those of Figures 2a, 4e, and 5a are shown in the supplemental information.
References
Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 2014; 28: 1472–1477.
Hitchcock IS, Kaushansky K . Thrombopoietin from beginning to end. Br J Haematol 2014; 165: 259–268.
Ku H, Yonemura Y, Kaushansky K, Ogawa M . Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood 1996; 87: 4544–4551.
Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y et al. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood 1995; 86: 4025–4033.
Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood 1997; 90: 4369–4383.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270.
Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M . Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009; 114: 3748–3756.
Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F . Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood 2002; 100: 3495–3503.
Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 2007; 110: 986–993.
Pilling D, Buckley CD, Salmon M, Gomer RH . Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol 2003; 171: 5537–5546.
Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med 2016; 213: 1723–1740.
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A . Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994; 1: 71–81.
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN . Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001; 166: 7556–7562.
Pilling D, Fan T, Huang D, Kaul B, Gomer RH . Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 2009; 4: e7475.
Mehrad B, Strieter RM . Fibrocytes and the pathogenesis of diffuse parenchymal lung disease. Fibrogenesis Tissue Repair 2012; 5: S22.
Keeley EC, Mehrad B, Strieter RM . The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair 2011; 4: 2.
Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006; 45: 429–438.
Reich B, Schmidbauer K, Rodriguez Gomez M, Johannes Hermann F, Gobel N, Bruhl H et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int 2013; 84: 78–89.
Reilkoff RA, Bucala R, Herzog EL . Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011; 11: 427–435.
Yanai N, Suzuki M, Obinata M . Hepatocyte cell lines established from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Exp Cell Res 1991; 197: 50–56.
Obinata M . Conditionally immortalized cell lines with differentiated functions established from temperature-sensitive T-antigen transgenic mice. Genes Cells 1997; 2: 235–244.
Crawford JR, Pilling D, Gomer RH . Improved serum-free culture conditions for spleen-derived murine fibrocytes. J Immunol Methods 2010; 363: 9–20.
Pilling D, Vakil V, Gomer RH . Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods 2009; 351: 62–70.
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A . European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005; 90: 1128–1132.
Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010; 115: 3109–3117.
Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS . The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood 2014; 124: 3956–3963.
Wang X, Haylock D, Hu CS, Kowalczyk W, Jiang T, Qiu J et al. A thrombopoietin receptor antagonist is capable of depleting myelofibrosis hematopoietic stem and progenitor cells. Blood 2016; 127: 3398–3409.
Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med 2013; 19: 437–445.
Hashimoto M, Nasser H, Bhuyan F, Kuse N, Satou Y, Harada S et al. Fibrocytes differ from macrophages but can be infected with HIV-1. J Immunol 2015; 195: 4341–4350.
Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica 2016; 101: 660–671.
Mascarenhas J, Li T, Sandy L, Newsom C, Petersen B, Godbold J et al. Anti-transforming growth factor-beta therapy in patients with myelofibrosis. Leuk Lymphoma 2014; 55: 450–452.
Mesa RA, Tefferi A, Elliott MA, Hoagland HC, Call TG, Schroeder GS et al. A phase II trial of pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone), a novel anti-fibrosing agent, in myelofibrosis with myeloid metaplasia. Br J Haematol 2001; 114: 111–113.
Crawford JR, Pilling D, Gomer RH . FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol 2012; 92: 699–711.
Duffield JS, Lupher ML Jr . PRM-151 (recombinant human serum amyloid P/pentraxin 2) for the treatment of fibrosis. Drug News Perspect 2010; 23: 305–315.
Veillette A, Guo H . CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol 2013; 88: 168–177.
Jakubowiak A, Offidani M, Pegourie B, De La Rubia J, Garderet L, Laribi K et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016; 127: 2833–2840.
Wang JC, Chen C, Lou LH, Mora M . Blood thrombopoietin, IL-6 and IL-11 levels in patients with agnogenic myeloid metaplasia. Leukemia 1997; 11: 1827–1832.
Cerutti A, Custodi P, Duranti M, Noris P, Balduini CL . Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol 1997; 99: 281–284.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maekawa, T., Osawa, Y., Izumi, T. et al. Myeloproliferative leukemia protein activation directly induces fibrocyte differentiation to cause myelofibrosis. Leukemia 31, 2709–2716 (2017). https://doi.org/10.1038/leu.2017.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.112
This article is cited by
-
Continuous Indexing of Fibrosis (CIF): improving the assessment and classification of MPN patients
Leukemia (2023)
-
Inhibition of ERK1/2 signaling prevents bone marrow fibrosis by reducing osteopontin plasma levels in a myelofibrosis mouse model
Leukemia (2023)
-
GLI1 activates pro-fibrotic pathways in myelofibrosis fibrocytes
Cell Death & Disease (2022)
-
Neoplastic fibrocytes play an essential role in bone marrow fibrosis in Jak2V617F-induced primary myelofibrosis mice
Leukemia (2021)
-
Calreticulin del52 and ins5 knock-in mice recapitulate different myeloproliferative phenotypes observed in patients with MPN
Nature Communications (2020)