Abstract
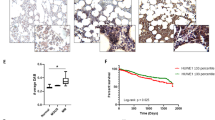
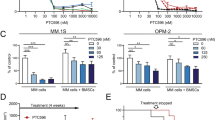
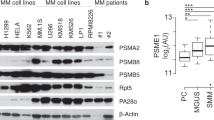
Proteasome inhibitor bortezomib is an effective therapy for relapsed and newly diagnosed multiple myeloma (MM); however, dose-limiting toxicities and the development of resistance can limit its long-term utility. Recent research has focused on targeting ubiquitin receptors upstream of 20S proteasome, with the aim of generating less toxic therapies. Here we show that 19S proteasome-associated ubiquitin receptor Rpn13 is more highly expressed in MM cells than in normal plasma cells. Rpn13-siRNA (small interfering RNA) decreases MM cell viability. A novel agent RA190 targets Rpn13 and inhibits proteasome function, without blocking the proteasome activity or the 19S deubiquitylating activity. CRISPR/Cas9 Rpn13-knockout demonstrates that RA190-induced activity is dependent on Rpn13. RA190 decreases viability in MM cell lines and patient MM cells, inhibits proliferation of MM cells even in the presence of bone marrow stroma and overcomes bortezomib resistance. Anti-MM activity of RA190 is associated with induction of caspase-dependent apoptosis and unfolded protein response-related apoptosis. MM xenograft model studies show that RA190 is well tolerated, inhibits tumor growth and prolongs survival. Combining RA190 with bortezomib, lenalidomide or pomalidomide induces synergistic anti-MM activity. Our preclinical data validates targeting Rpn13 to overcome bortezomib resistance, and provides the framework for clinical evaluation of Rpn13 inhibitors, alone or in combination, to improve patient outcome in MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Adams J . The proteasome: a suitable antineoplastic target. Nat Rev Cancer 2004; 4: 349–360.
Chauhan D, Hideshima T, Anderson KC . Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol 2005; 45: 465–476.
Goldberg AL . Protein degradation and protection against misfolded or damaged proteins. Nature 2003; 426: 895–899.
Hershko A . The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angew Chem 2005; 44: 5932–5943.
Finley D . Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 2009; 78: 477–513.
Pickart CM, Eddins MJ . Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta 2004; 1695: 55–72.
Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 2008; 453: 481–488.
Schreiner P, Chen X, Husnjak K, Randles L, Zhang N, Elsasser S et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature 2008; 453: 548–552.
Elsasser S, Finley D . Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol 2005; 7: 742–749.
Zhu Q, Wani G, Wang QE, El-mahdy M, Snapka RM, Wani AA . Deubiquitination by proteasome is coordinated with substrate translocation for proteolysis in vivo. Exp Cell Res 2005; 307: 436–451.
Bhattacharyya S, Yu H, Mim C, Matouschek A . Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol 2014; 15: 122–133.
Kane RC, Bross PF, Farrell AT, Pazdur R . Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist 2003; 8: 508–513.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–2617.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D et al. Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood 2005; 106: 2977–2981.
Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012; 120: 2817–2825.
Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol 2012; 158: 739–748.
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 2006; 24: 3113–3120.
Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood 2005; 106: 3777–3784.
Huber EM, Heinemeyer W, Groll M . Bortezomib-resistant mutant proteasomes: structural and biochemical evaluation with carfilzomib and ONX 0914. Structure 2015; 23: 407–417.
Cai X, Bhattacharyya S, Plitt A, Raibagkar P, LaBuzetta JN, Schleicher SM et al. Management of Posterior reversible encephalopathy syndrome induced by carfilzomib in a patient with multiple myeloma. J Clin Oncol 2016; 34: e1–e5.
Wanchoo R, Khan S, Kolitz JE, Jhaveri KD . Carfilzomib-related acute kidney injury may be prevented by N-acetyl-l-cysteine. J Oncol Pharm Pract 2015; 21: 313–316.
Harvey RD . Incidence and management of adverse events in patients with relapsed and/or refractory multiple myeloma receiving single-agent carfilzomib. Clin Pharmacol 2014; 6: 87–96.
Atrash S, Tullos A, Panozzo S, Bhutani M, Van Rhee F, Barlogie B et al. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J 2015; 5: e272.
Tian Z, D'Arcy P, Wang X, Ray A, Tai YT, Hu Y et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 2014; 123: 706–716.
Anchoori RK, Karanam B, Peng S, Wang JW, Jiang R, Tanno T et al. A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer. Cancer Cell 2013; 24: 791–805.
Trader DJ, Simanski S, Kodadek T . A reversible and highly selective inhibitor of the proteasomal ubiquitin receptor rpn13 is toxic to multiple myeloma cells. J Am Chem Soc 2015; 137: 6312–6319.
Qiu XB, Ouyang SY, Li CJ, Miao S, Wang L, Goldberg AL . hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J 2006; 25: 5742–5753.
Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol 2006; 8: 994–1002.
Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 2009; 16: 309–323.
D'Arcy P, Brnjic S, Olofsson MH, Fryknas M, Lindsten K, De Cesare M et al. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 2011; 17: 1636–1640.
Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell 2005; 8: 407–419.
Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 2012; 22: 345–358.
Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood 2008; 111: 1654–1664.
Chauhan D, Ray A, Viktorsson K, Spira J, Paba-Prada C, Munshi N et al. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res 2013; 19: 3019–3031.
Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP . Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet 2005; 14: 2787–2799.
Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 2010; 467: 179–184.
Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL . A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J 2001; 20: 5187–5196.
Verma R, Aravind L, Oania R, McDonald WH, Yates JR 3rd, Koonin EV et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26 S proteasome. Science 2002; 298: 611–615.
Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol 2002; 9: 1149–1159.
Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood 1996; 87: 1104–1112.
Ray A, Das DS, Song Y, Richardson P, Munshi NC, Chauhan D et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015; 29: 1441–1444.
Das DS, Ray A, Song Y, Richardson P, Trikha M, Chauhan D et al. Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD((R)) immunomodulatory drug pomalidomide. Br J Haematol 2015; 171: 798–812.
Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L et al. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy 2014; 10: 1380–1390.
Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R . Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia 2004; 6: 744–750.
Fejzo MS, Dering J, Ginther C, Anderson L, Ramos L, Walsh C et al. Comprehensive analysis of 20q13 genes in ovarian cancer identifies ADRM1 as amplification target. Genes Chromosomes Cancer 2008; 47: 873–883.
Chen W, Hu XT, Shi QL, Zhang FB, He C . Knockdown of the novel proteasome subunit Adrm1 located on the 20q13 amplicon inhibits colorectal cancer cell migration, survival and tumorigenicity. Oncol Rep 2009; 21: 531–537.
Carvalho B, Postma C, Mongera S, Hopmans E, Diskin S, van de Wiel MA et al. Multiple putative oncogenes at the chromosome 20q amplicon contribute to colorectal adenoma to carcinoma progression. Gut 2009; 58: 79–89.
Yang X, Miao X, Wen Y, Hu J, Dai W, Yin B . A possible connection between adhesion regulating molecule 1 overexpression and nuclear factor kappa B activity in hepatocarcinogenesis. Oncol Rep 2012; 28: 283–290.
Bergsagel PL, Kuehl WM . Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol 2005; 23: 6333–6338.
Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM . Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA 1996; 93: 13931–13936.
Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T et al. Characterization of the MM.1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol 2003; 31: 271–282.
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 2007; 109: 3489–3495.
Burger R, Guenther A, Bakker F, Schmalzing M, Bernand S, Baum W et al. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J 2001; 2: 42–53.
Acknowledgements
This investigation was supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) Grant P50100707, PO1-CA078378 and RO1 CA050947. KCA is an American Cancer Society Clinical Research Professor.
Author contributions
YS performed the experiments, interpreted data and wrote the manuscript; RDC helped in immunohistochemistry experiments; SL helped in reverse transcription-PCR studies; AR and DS helped in animal studies; YT provided clinical samples; DC designed research, analyzed data and wrote the manuscript; KCA analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, Y., Ray, A., Li, S. et al. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia 30, 1877–1886 (2016). https://doi.org/10.1038/leu.2016.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.97
This article is cited by
-
A drug repurposing strategy for overcoming human multiple myeloma resistance to standard-of-care treatment
Cell Death & Disease (2022)
-
Proteomic analysis identifies mechanism(s) of overcoming bortezomib resistance via targeting ubiquitin receptor Rpn13
Leukemia (2021)
-
Identification of novel anti-tumor therapeutic target via proteomic characterization of ubiquitin receptor ADRM1/Rpn13
Blood Cancer Journal (2021)
-
Structure-guided bifunctional molecules hit a DEUBAD-lacking hRpn13 species upregulated in multiple myeloma
Nature Communications (2021)
-
Unveiling the immunomodulatory properties of Haemonchus contortus adhesion regulating molecule 1 interacting with goat T cells
Parasites & Vectors (2020)