Abstract
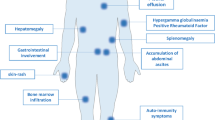
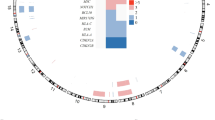
Intravascular large B-cell lymphoma (IVLBCL) is a distinct disease entity with the peculiar characteristic that tumor cells proliferate within vessels. Despite recent advances in understanding the disease from clinical aspects, the underlying pathogenesis remains unknown. Here we demonstrate analyses of IVLBCL biology using four xenograft mouse models established from primary IVLBCL samples. In all four models, the main characteristic of IVLBCL tumor cell proliferation within vessels was retained. Time-lapse engraftment analyses revealed that the tumor cells initially engrafted and proliferated in the sinusoids and vessels in the liver and then engrafted and proliferated in multiple organs. Intriguingly, serial passage of tumor cells from the adrenal gland of a transplanted mouse developed from primary patient bone marrow cells into a second mouse showed that the tumor cells mainly distributed into the adrenal gland in the second mouse, implying the existence of clonal selection and/or evolution at engraftment of a specific organ. Gene expression profiling analyses demonstrated that the gene set associated with cell migration was enriched for normal peripheral blood B cells, indicating that inhibition of cell migration might be involved in IVLBCL pathogenesis. In conclusion, the mouse xenograft models described here are essential tools for uncovering IVLBCL biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Nakamura S, Ponzoni M, Campo E . Intravascular large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al (eds), WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008; pp 252–253.
Ponzoni M, Ferreri AJ, Campo E, Facchetti F, Mazzucchelli L, Yoshino T et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol 2007; 25: 3168–3173.
Ferreri AJ, Campo E, Ambrosetti A, Ilariucci F, Seymour JF, Willemze R et al. Anthracycline-based chemotherapy as primary treatment for intravascular lymphoma. Ann Oncol 2004; 15: 1215–1221.
Murase T, Yamaguchi M, Suzuki R, Okamoto M, Sato Y, Tamaru J et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood 2007; 109: 478–485.
Gill S, Melosky B, Haley L, ChanYan C . Use of random skin biopsy to diagnose intravascular lymphoma presenting as fever of unknown origin. Am J Med 2003; 114: 56–58.
Asada N, Odawara J, Kimura S, Aoki T, Kamakura M, Takeuchi M et al. Use of random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc 2007; 82: 1525–1527.
Matsue K, Asada N, Odawara J, Aoki T, Kimura S, Iwama K et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol 2011; 90: 417–421.
Shimada K, Matsue K, Yamamoto K, Murase T, Ichikawa N, Okamoto M et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol 2008; 26: 3189–3195.
Ferreri AJ, Dognini GP, Bairey O, Szomor A, Montalban C, Horvath B et al. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in 'Western' patients with intravascular large B-cell lymphoma. Br J Haematol 2008; 143: 253–257.
Shimada K, Kinoshita T, Naoe T, Nakamura S . Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol 2009; 10: 895–902.
Ponzoni M, Arrigoni G, Gould VE, Del Curto B, Maggioni M, Scapinello A et al. Lack of CD 29 (beta1 integrin) and CD 54 (ICAM-1) adhesion molecules in intravascular lymphomatosis. Hum Pathol 2000; 31: 220–226.
Kusaba T, Hatta T, Tanda S, Kameyama H, Tamagaki K, Okigaki M et al. Histological analysis on adhesive molecules of renal intravascular large B cell lymphoma treated with CHOP chemotherapy and rituximab. Clin Nephrol 2006; 65: 222–226.
Morton CL, Houghton PJ . Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2007; 2: 247–250.
Tomita A, Hiraga J, Kiyoi H, Ninomiya M, Sugimoto T, Ito M et al. Epigenetic regulation of CD20 protein expression in a novel B-cell lymphoma cell line, RRBL1, established from a patient treated repeatedly with rituximab-containing chemotherapy. Int J Hematol 2007; 86: 49–57.
Dewan MZ, Watanabe M, Ahmed S, Terashima K, Horiuchi S, Sata T et al. Hodgkin's lymphoma cells are efficiently engrafted and tumor marker CD30 is expressed with constitutive nuclear factor-kappaB activity in unconditioned NOD/SCID/gammac(null) mice. Cancer Sci 2005; 96: 466–473.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002; 100: 3175–3182.
Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 2007; 21: 136–142.
van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17: 2257–2317.
Katakai T, Hara T, Sugai M, Gonda H, Shimizu A . Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med 2004; 200: 783–795.
Tagawa H, Tsuzuki S, Suzuki R, Karnan S, Ota A, Kameoka Y et al. Genome-wide array-based comparative genomic hybridization of diffuse large B-cell lymphoma: comparison between CD5-positive and CD5-negative cases. Cancer Res 2004; 64: 5948–5955.
Hocking TD, Boeva V, Rigaill G, Schleiermacher G, Janoueix-Lerosey I, Delattre O et al. SegAnnDB: interactive Web-based genomic segmentation. Bioinformatics 2014; 30: 1539–1546.
Eisen MB, Spellman PT, Brown PO, Botstein D . Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998; 95: 14863–14868.
Tusher VG, Tibshirani R, Chu G . Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001; 98: 5116–5121.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Murase T, Nakamura S, Kawauchi K, Matsuzaki H, Sakai C, Inaba T et al. An Asian variant of intravascular large B-cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B-cell lymphoma associated with haemophagocytic syndrome. Br J Haematol 2000; 111: 826–834.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–282.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511.
Suguro M, Yoshida N, Umino A, Kato H, Tagawa H, Nakagawa M et al. Clonal heterogeneity of lymphoid malignancies correlates with poor prognosis. Cancer Sci 2014; 105: 897–904.
Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–1947.
Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR . Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10: 778–790.
Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285: 895–898.
Acknowledgements
We thank Mr Kuniyoshi Kitou, Ms Kazuko Matsuba, Ms Yuko Katayama and Mr Yoshiaki Inagaki (Nagoya University) for immunohistochemistry work; Ms Asako Watanabe, Ms Yoko Matsuyama, Ms Chika Wakamatsu, Ms Manami Kira, Ms Rie Kojima, Ms Yukie Konishi, Ms Yuko Kojima and Ms Emi Kohno (Nagoya University) for assistance with laboratory work; Dr Seitaro Terakura (Nagoya University), Dr Kuniaki Kitamura and Dr Eri Iwata (Ichinomiya Municipal Hospital) for providing patient samples and information; Dr Tomoya Katakai (Niigata University) for providing the BLS4 cell line; Mr Toshiyuki Takeuchi (Oncomics Co. Ltd) for providing information regarding GEP analysis; and Ms Kyoko Hirano (Aichi Cancer Center) for assistance with the array CGH study. This work was supported by the Program to Disseminate Tenure Tracking System, MEXT, Japan, by a JSPS Grant-in-Aid for Young Scientists (B) 26860724, by the Practical Research for Innovative Cancer Control, MHLW/AMED, Japan, and by the Kanae Foundation for the Promotion of Medical Science grant to KS.
Author contributions
K Shimada, TN, FH, M Seto, AT and HK designed research; K Shimada, K Sugimoto, TT, YT, AS and TA established mouse models; K Shimada, SS, K Sugimoto and YT performed experiments; K Shimada, MN, M Suguro, AH, IT, SN and M Seto analyzed and interpreted data; K Shimada, MN, M Suguro, AH, TDH and IT performed statistical analysis; TN, SN, FH, M Seto, AT and HK supervised research; K Shimada, MN, M Suguro, AH, IT, M Seto, AT and HK wrote the manuscript; and all authors reviewed and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K Sugimoto is an employee of Otsuka Pharmaceutical Co., Ltd and M Seto is an employee of Immuno-Biological Laboratories Co., Ltd. TN received research funding from Astellas Pharma Inc., Celgene KK, Fujifilm Corporation, Kyowa-Hakko Kirin Co., Ltd, Nippon Boehringer Ingelheim Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Pfizer Inc. and Toyoma Chemical Co., Ltd, has issued patents and royalties with Chugai Pharmaceutical Co., Ltd, and Kyowa-Hakko Kirin Co., Ltd and has pending patents and royalties with Fujifilm Corporation. HK has served as a consultant for Astellas Pharma Inc. and Kyowa-Hakko Kirin Co., Ltd and has received research funding from Fujifilm Corporation, Nippon Boehringer Ingelheim Co., Ltd, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd, Kyowa-Hakko Kirin Co., Ltd, Zenyaku Kogyo Company Ltd, FUJIFILM RI Pharma Co., Ltd, Nippon Shinyaku Co., Ltd, the Japan Blood Products Organization, Astellas Pharma Inc., Eisai Co., Ltd, Yakult Honsha Co., Ltd, Pfizer Inc., Takeda Pharmaceutical Co., Ltd, MSD K.K., Alexion Pharmaceuticals, Teijin Ltd, Mochida Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd and Novartis Pharma K.K. and has patents and royalties with Fujifilm Corporation.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Shimada, K., Shimada, S., Sugimoto, K. et al. Development and analysis of patient-derived xenograft mouse models in intravascular large B-cell lymphoma. Leukemia 30, 1568–1579 (2016). https://doi.org/10.1038/leu.2016.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.67
This article is cited by
-
Clinicopathological characteristics associated with the engraftment of patient lymphoma cells in NOG mice
International Journal of Hematology (2023)
-
Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma
Oncogene (2021)
-
Development and Significance of Mouse Models in Lymphoma Research
Current Hematologic Malignancy Reports (2019)
-
Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies
European Journal of Nuclear Medicine and Molecular Imaging (2017)