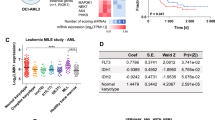
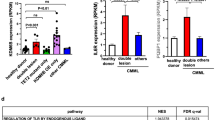
Abstract
Both membrane-proximal and truncation mutations in CSF3R have recently been reported to drive the onset of chronic neutrophilic leukemia (CNL). Here we show that although truncation mutation alone cannot induce leukemia, both proximal and compound mutations (proximal and truncation mutations on same allele) are leukemogenic with a disease latency of 90 and 23 days, respectively. Comparative whole-genome expression profiling and biochemical experiments revealed that induced expression of Mapk adaptor protein Ksr1 and enhanced Mapk signaling are crucial to leukemogenesis by CSF3R proximal and compound mutants. Moreover, inhibition of Mek1/2 by trametinib alone is sufficient to suppress leukemia induced by both CSF3R proximal and ruxolitinib-resistant compound mutations. Together, these findings elucidate a Mapk-dependent mechanism of CSF3R-induced pathogenesis, and they establish the rationale for clinical evaluation of MEK1/2 inhibition in CNL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Daley GQ, Van Etten RA, Baltimore D . Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 1990; 247: 824–830.
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996; 2: 561–566.
Krause DS, Van Etten RA . Tyrosine kinases as targets for cancer therapy. N Engl J Med 2005; 353: 172–187.
Sawyers C . Targeted cancer therapy. Nature 2004; 432: 294–297.
Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med 2013; 368: 1781–1790.
Pardanani A, Lasho TL, Laborde RR, Elliott M, Hanson CA, Knudson RA et al. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia 2013; 27: 1870–1873.
Dong F, Brynes RK, Tidow N, Welte K, Lowenberg B, Touw IP . Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med 1995; 333: 487–493.
Dong F, Hoefsloot LH, Schelen AM, Broeders CA, Meijer Y, Veerman AJ et al. Identification of a nonsense mutation in the granulocyte-colony-stimulating factor receptor in severe congenital neutropenia. Proc Natl Acad Sci USA 1994; 91: 4480–4484.
Hunter MG, Avalos BR . Phosphatidylinositol 3'-kinase and SH2-containing inositol phosphatase (SHIP) are recruited by distinct positive and negative growth-regulatory domains in the granulocyte colony-stimulating factor receptor. J Immunol 1998; 160: 4979–4987.
Hortner M, Nielsch U, Mayr LM, Johnston JA, Heinrich PC, Haan S . Suppressor of cytokine signaling-3 is recruited to the activated granulocyte-colony stimulating factor receptor and modulates its signal transduction. J Immunol 2002; 169: 1219–1227.
Hunter MG, Jacob A, O'Donnell LC, Agler A, Druhan LJ, Coggeshall KM et al. Loss of SHIP and CIS recruitment to the granulocyte colony-stimulating factor receptor contribute to hyperproliferative responses in severe congenital neutropenia/acute myelogenous leukemia. J Immunol 2004; 173: 5036–5045.
Ward AC, Oomen SP, Smith L, Gits J, van Leeuwen D, Soede-Bobok AA et al. The SH2 domain-containing protein tyrosine phosphatase SHP-1 is induced by granulocyte colony-stimulating factor (G-CSF) and modulates signaling from the G-CSF receptor. Leukemia 2000; 14: 1284–1291.
van de Geijn GJ, Gits J, Aarts LH, Heijmans-Antonissen C, Touw IP . G-CSF receptor truncations found in SCN/AML relieve SOCS3-controlled inhibition of STAT5 but leave suppression of STAT3 intact. Blood 2004; 104: 667–674.
Aarts LH, Roovers O, Ward AC, Touw IP . Receptor activation and 2 distinct COOH-terminal motifs control G-CSF receptor distribution and internalization kinetics. Blood 2004; 103: 571–579.
Futami M, Zhu QS, Whichard ZL, Xia L, Ke Y, Neel BG et al. G-CSF receptor activation of the Src kinase Lyn is mediated by Gab2 recruitment of the Shp2 phosphatase. Blood 2011; 118: 1077–1086.
Germeshausen M, Ballmaier M, Welte K . Implications of mutations in hematopoietic growth factor receptor genes in congenital cytopenias. Ann N Y Acad Sci. 2001; 938: 305–320.
Touw IP, Beekman R . Severe congenital neutropenia and chronic neutrophilic leukemia: an intriguing molecular connection unveiled by oncogenic mutations in CSF3R. Haematologica 2013; 98: 1490–1492.
Carapeti M, Soede-Bobok A, Hochhaus A, Sill H, Touw IP, Goldman JM et al. Rarity of dominant-negative mutations of the G-CSF receptor in patients with blast crisis of chronic myeloid leukemia or de novo acute leukemia. Leukemia 1997; 11: 1005–1008.
Hermans MH, Antonissen C, Ward AC, Mayen AE, Ploemacher RE, Touw IP . Sustained receptor activation and hyperproliferation in response to granulocyte colony-stimulating factor (G-CSF) in mice with a severe congenital neutropenia/acute myeloid leukemia-derived mutation in the G-CSF receptor gene. J Exp Med 1999; 189: 683–692.
Hunter MG, Avalos BR . Granulocyte colony-stimulating factor receptor mutations in severe congenital neutropenia transforming to acute myelogenous leukemia confer resistance to apoptosis and enhance cell survival. Blood 2000; 95: 2132–2137.
McLemore ML, Poursine-Laurent J, Link DC . Increased granulocyte colony-stimulating factor responsiveness but normal resting granulopoiesis in mice carrying a targeted granulocyte colony-stimulating factor receptor mutation derived from a patient with severe congenital neutropenia. J Clin Invest 1998; 102: 483–492.
Mitsui T, Watanabe S, Taniguchi Y, Hanada S, Ebihara Y, Sato T et al. Impaired neutrophil maturation in truncated murine G-CSF receptor-transgenic mice. Blood 2003; 101: 2990–2995.
Maxson JE, Luty SB, MacManiman JD, Abel ML, Druker BJ, Tyner JW . Ligand independence of the T618I mutation in the colony-stimulating factor 3 receptor (CSF3R) protein results from loss of O-linked glycosylation and increased receptor dimerization. J Biol Chem 2014; 289: 5820–5827.
Fleischman AG, Maxson JE, Luty SB, Agarwal A, Royer LR, Abel ML et al. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood 2013; 122: 3628–3631.
Gotlib J, Maxson JE, George TI, Tyner JW . The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood 2013; 122: 1707–1711.
Kesarwani M, Huber E, Kincaid Z, Evelyn CR, Biesiada J, Rance M et al. Targeting substrate-site in Jak2 kinase prevents emergence of genetic resistance. Sci Rep 2015; 5: 14538.
Komurov K, Dursun S, Erdin S, Ram PT . NetWalker: a contextual network analysis tool for functional genomics. BMC Genomics 2012; 13: 282.
Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC . Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity 1996; 5: 491–501.
Kawakami M, Tsutsumi H, Kumakawa T, Abe H, Hirai M, Kurosawa S et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood 1990; 76: 1962–1964.
Nicholson SE, Oates AC, Harpur AG, Ziemiecki A, Wilks AF, Layton JE . Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. Proc Natl Acad Sci USA 1994; 91: 2985–2988.
Nicholson SE, Novak U, Ziegler SF, Layton JE . Distinct regions of the granulocyte colony-stimulating factor receptor are required for tyrosine phosphorylation of the signaling molecules JAK2, Stat3, and p42, p44MAPK. Blood 1995; 86: 3698–3704.
Corey SJ, Burkhardt AL, Bolen JB, Geahlen RL, Tkatch LS, Tweardy DJ . Granulocyte colony-stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc Natl Acad Sci USA 1994; 91: 4683–4687.
Tian SS, Tapley P, Sincich C, Stein RB, Rosen J, Lamb P . Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5, and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood 1996; 88: 4435–4444.
Shimoda K, Feng J, Murakami H, Nagata S, Watling D, Rogers NC et al. Jak1 plays an essential role for receptor phosphorylation and Stat activation in response to granulocyte colony-stimulating factor. Blood 1997; 90: 597–604.
Ward AC, Monkhouse JL, Csar XF, Touw IP, Bello PA . The Src-like tyrosine kinase Hck is activated by granulocyte colony-stimulating factor (G-CSF) and docks to the activated G-CSF receptor. Biochem Biophys Res Commun 1998; 251: 117–123.
Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ . G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 2006; 107: 1847–1856.
Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M et al. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol 2002; 22: 3035–3045.
Lozano J, Xing R, Cai Z, Jensen HL, Trempus C, Mark W et al. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res 2003; 63: 4232–4238.
Xing HR, Cordon-Cardo C, Deng X, Tong W, Campodonico L, Fuks Z et al. Pharmacologic inactivation of kinase suppressor of ras-1 abrogates Ras-mediated pancreatic cancer. Nat Med 2003; 9: 1266–1268.
Acknowledgements
We are thankful to Jose Cancelas for critical reading and suggestions. This study was supported by grants to MA from NCI (1RO1CA155091).
Author contributions
MK, SR, ZK and MA designed and performed experiments and analyzed data. EH, JL, YK and ZS performed in vitro and in vivo experiments. KK analyzed the gene expression data. LG provided intellectual insights and help in designing the experiments. MK and MA wrote the paper. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Rohrabaugh, S., Kesarwani, M., Kincaid, Z. et al. Enhanced MAPK signaling is essential for CSF3R-induced leukemia. Leukemia 31, 1770–1778 (2017). https://doi.org/10.1038/leu.2016.376
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.376
This article is cited by
-
MAPK-negative feedback regulation confers dependence to JAK2V617F signaling
Leukemia (2023)
-
Current Management of Chronic Neutrophilic Leukemia
Current Treatment Options in Oncology (2021)
-
Time resolved quantitative phospho-tyrosine analysis reveals Bruton’s Tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors
Leukemia (2019)
-
Chronic neutrophilic leukemia: new science and new diagnostic criteria
Blood Cancer Journal (2018)
-
Recent Progress in Chronic Neutrophilic Leukemia and Atypical Chronic Myeloid Leukemia
Current Hematologic Malignancy Reports (2017)