Abstract
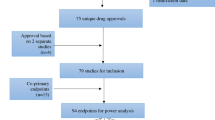
To minimize adverse events (AEs) unrelated to drugs and maximize the likelihood of drug approvals, eligibility criteria for randomized controlled trials (RCTs) may be overly restrictive. The purpose of this study was to determine if RCTs in hematologic malignancies exclude patients irrespective of known toxicities or observed AEs. MEDLINE was searched from 1/2010 to 1/2015 for RCTs published in high-impact journals. Of 97 trials, 33% were conducted in leukemia, 28% in lymphoma, 34% in multiple myeloma and 5% in myelodysplastic syndromes or myelofibrosis. Expected toxicities at thresholds of ⩾10%, ⩾5% and <5% were not correlated with cardiac, hepatic or renal eligibility criteria (logistic regression). To explore this lack of correlation we tested the concordance of expected toxicities and eligibility criteria using a modified version of McNemar’s test: at each threshold, hepatic, renal and cardiac expected toxicities were significantly discordant with eligibility criteria. Hepatic and renal eligibility criteria were also not correlated with observed AEs, P=0.69 and P=0.77, respectively, but a significant correlation was detected between cardiac eligibility criteria and observed AEs, P=0.02. Thus, the analyzed RCTs excluding patients with organ dysfunction do not reflect expected toxicities, based on prescription drug labels/prior experience, or reported AEs on the trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Van Spall HG, Toren A, Kiss A, Fowler RA . Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007; 297: 1233–1240.
Fuks A, Weijer C, Freedman B, Shapiro S, Skrutkowska M, Riaz A . A study in contrasts: eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J Clin Epidemiol 1998; 51: 69–79.
Guyatt GH, Haynes RB, Jaeschke RZ, Cook DJ, Green L, Naylor CD et al. Users' Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users' Guides to patient care. Evidence-Based Medicine Working Group. JAMA 2000; 284: 1290–1296.
Bates SE, Berry DA, Balasubramaniam S, Bailey S, LoRusso RM, Rubin EH . Advancing Clinical Trials to Streamline Drug Development. Clin Cancer Res 2015; 21: 4527–4535.
Lu D, Lu T, Stroh M, Graham RA, Agarwal P, Musib L et al. A survey of new oncology drug approvals in the USA from 2010 to 2015: a focus on optimal dose and related postmarketing activities. Cancer Chemother Pharmacol 2016; 77: 459–476.
Rubin EH, Gilliland DG . Drug development and clinical trials–the path to an approved cancer drug. Nat Rev Clin Oncol 2012; 9: 215–222.
Kim ES, Bernstein D, Hilsenbeck SG, Chung CH, Dicker AP, Ersek JL et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 2015; 33: 2815–2820.
George SL . Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol 1996; 14: 1364–1370.
Gerber DE, Pruitt SL, Halm EA . Should criteria for inclusion in cancer clinical trials be expanded. J Comp Eff Res 2015; 4: 289–291.
Clarey J, Kao SC, Clarke SJ, Vardy J . The eligibility of advanced non-small-cell lung cancer patients for targeted therapy clinical trials. Ann Oncol 2012; 23: 1229–1233.
Clement-Duchne C, Carnin C, Martinet Y . Too restrictive exclusion criteria in advanced stage lung cancer therapeutic trials: are we missing the target [letter]. J Thorac Oncol 2008; 3: 943.
Vardy J, Dadasovich R, Beale P, Boyer M, Clark SJ . Eligibility of patients with advanced non-small cell lung cancer for phase III chemotherapy trials. BMC Cancer 2009; 9: 130.
Impact Factor List 2014. Citfactor Website; 2014 Aug (cited on 8 Nov 2014). Available from http://www.citefactor.org/journal-impact-factor-list-2014.html.
2014 Journal Citation Reports. (Thomson Reuters, 2014); 2015 Jun (cited on 2 Mar 2016). Available from http://ipscience.thomsonreuters.com/product/journal-citationreports/?utm_source=false&utm_medium=false&utm_campaign=false.
American Society of Clinical Oncology. The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J Oncol Pract 2014; 10: 119–142.
Mandelblatt JS, Yabroff KR, Kerner JF . Equitable access to cancer services: a review of barriers to quality care. Cancer 1999; 86: 2378–2390.
Lara Jr PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol 2001; 19: 1728–1733.
Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 2002; 20: 2109–2117.
Cheng SK, Dietrich MS, Dilts DM . A sense of urgency. Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin Cancer Res 2010; 16: 5557–5563.
Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Clin Oncol 2013; 9: 267–276.
Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F . Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer 2002; 95: 1577–1573.
Jacobs SR, Weiner BJ, Reeve BB, Weinberger M, Minasian LM, Good MJ . Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin Trials 2014; 11: 565–575.
Kanarek NF, Kanarek MS, Olatoye D, Carducci MA . Removing barriers to participation in clinical trials, a conceptual framework and retrospective chart review study. Trials 2012; 13: 237.
Lemieux J, Goodwin PJ, Pritchard KI, Gelmon KA, Bordeleau LJ, Duchesne T et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol 2008; 26: 4457–4465.
Davis S, Wright PW, Schulman SF, Hill LK, Pinkham RD, Johnson LP et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer 1985; 56: 1710–1718.
Vist GE, Hagen KB, Deveraux PJ, Bryant D, Kristoffersen DT, Oxman AD . Outcomes of patients who participate in randomized controlled trials versus those of similar patients who do not participate. Cochrane Database Syst Rev 2007; 18: MR000009.
Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551.
Mamdani M, Juurlink DN, Lee DS, Rochon PA, Kopp A, Nagile G et al. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 2004; 363: 1751–1756.
Mao FJ, Rini BI . The ineligible patient: how to treat patients not included in clinical studies. World J Urol 2014; 32: 9–18.
Alberti P, Rossi E, Cornblath DR, Merkies IS, Postma TJ, Frigeni B et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neruotoxcity: two sides of the coin. Ann Oncl 2014; 25: 257–264.
Sivendran S, Latif A, McBride RB, Stensland KD, Wisnivesky J, Haines L et al. Adverse event reporting in cancer clinical trial publications. J Clin Oncol 2014; 32: 83–89.
Péron J, Maillet D, Gan HK, Chen EX, You B . Adherence to CONSORT adverse event reporting guidelines in randomized clinical trials evaluating systemic cancer therapy: a systematic review. J Clin Oncol 2013; 31: 3957–3963.
Maillet D, Blay JY, You B, Rachdi A, Gan HK, Péron J . The reporting of adverse events in oncology phase III trials: a comparison of the current status versus the expectations of the EORTC members. Ann Oncol 2015; 27: 192–198.
Mhaskar RS, Reljic T, Wao H, Kumar A, Miladinovic B, Djulbegovic B . Treatment-related harms: what was planned and what was reported? National Cancer Institute’s Co-operative group phase III randomized controlled trials: a systematic review. J Clin Epidemiol 2014; 67: 354–356.
Vera-Badillo FE, Shapiro R, Ocana A, Amir E, Tannock IF . Bias in reporting of end points of effi cacy and toxicity in randomized, clinical trials for women with breast cancer. Ann Oncol 2013; 24: 1238–1244.
Pitrou I, Boutron I, Ahmad N, Ravaud P . Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med 2009; 169: 1756–1761.
Jenkins M, Stone A, Jennison C . An adaptive seamless phase II/III design for oncology trials with subpopulation selection using corelated survivial endpoints. Pharm Stat 2011; 10: 347–356.
Bren L . Cancer Drugs: Weighing the Risks and Benefits. Cancer Treatment Watch; 2007 Jan (cited on 6 Jul 2016). Available from http://www.cancertreatmentwatch.org/general/drugs.shtml.
Basch E . The missing voice of patients in drug-safety reporting. N Engl J Med 2010; 362: 865–869.
Seruga B, Templeton AJ, Vera-Badillo FE, Ocana A, Amir E, Tannock IF . Under-reporting of harm in clincial trials. Lancet Oncol 2016; 17: e209–e219.
Berlin JA, Glasser SC, Ellenberg SS . Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Public Health 2008; 98: 1366–1371.
Institute of Medicine CoQoHCiA Crossing the quality chasm: a new health system for the 21st century. National Academy Press: Washington, DC, USA, 2001.
Scharf O, Colevas AD . Adverse event reproitng in publications compared with sposnor database for cancer clinical trials. J Clin Oncol 2006; 24: 2457–2465.
Acknowledgements
Dr Sekeres is funded, in part, by the Edward P Evans Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study has been presented, in part, at the 2015 American Society of Hematology annual meeting as an oral presentation on 7 December 2015 in Orlando, FL, USA.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Statler, A., Radivoyevitch, T., Siebenaller, C. et al. The relationship between eligibility criteria and adverse events in randomized controlled trials of hematologic malignancies. Leukemia 31, 1808–1815 (2017). https://doi.org/10.1038/leu.2016.374
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.374