Abstract
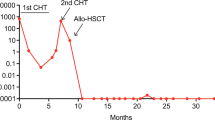
The APL0406 study showed that arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) are not inferior to standard ATRA and chemotherapy (CHT) in newly diagnosed, low–intermediaterisk acute promyelocytic leukaemia (APL). We analysed the kinetics of promyelocytic leukaemia–retinoic acid receptor-α (PML–RARα) transcripts by real-time quantitative PCR (RQ-PCR) in bone marrow samples from 184 patients and assessed the prognostic impact of fms-related tyrosine kinase 3–internal tandem duplication (FLT3–ITD) in 159 patients enrolled in this trial in Italy. After induction therapy, the reduction of PML–RARα transcripts was significantly greater in patients receiving ATRA-CHT as compared with those treated with ATRA–ATO (3.4 vs 2.9 logs; P=0.0182). Conversely, at the end of consolidation, a greater log reduction of PML–RARα transcripts was detected in the ATRA–ATO as compared with the ATRA–CHT group (6.3 vs 5.3 logs; P=0.0024). FLT3–ITD mutations had no significant impact on either event-free survival (EFS) or cumulative incidence of relapse in patients receiving ATRA–ATO, whereas a trend for inferior EFS was observed in FLT3–ITD-positive patients receiving ATRA-CHT. Our study shows at the molecular level that ATRA–ATO exerts at least equal and probably superior antileukaemic efficacy compared with ATRA–CHT in low–intermediaterisk APL. The data also suggest that ATRA–ATO may abrogate the negative prognostic impact of FLT3–ITD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Iland HJ, Wei A, Seymour JF . Have all-trans retinoic acid and arsenic trioxide replaced all-trans retinoic acid and anthracyclines in APL as standard of care. Best Pract Res Clin Haematol 2014; 27: 39–52.
Matthews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood 2006; 107: 2627–2632.
Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 2001; 19: 3852–3860.
Matthews V, Chendamarai E, George B, Viswabandya A, Svrivastava A . Treatment of acute promyelocytic leukemia with single agent arsenic trioxide. Mediterr J Hematol Infect Dis 2011; 3: e2011056.
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA 2004; 101: 5328–5335.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369: 111–121.
Chomczynski P, Sacchi N . Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159.
van Dongen JJM, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Leukemia 1999; 13: 1901–1928.
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Noguera NI, Breccia M, Divona M, Diverio D, Costa V, De Santis S et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia 2002; 16: 2185–2189.
Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N et al. Standardization and quality control studies of real-time quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia: a Europe Against Cancer Program. Leukemia 2003; 17: 2318–2357.
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol 2009; 27: 3650–3658.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Lo Coco F, Diverio D, Pandolfi PP, Biondi A, Rossi V, Avvisati G et al. Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukaemia. Lancet 1992; 340: 1437–1438.
Esteve J, Escoda L, Martín G,, Rubio V, Díaz-Mediavilla J, González M et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans-retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia 2007; 21: 446–452.
Santamaria C, Chillòn MC, Fernandez C, Martín-Jiménez P, Balanzategui A, García Sanz R et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica 2011; 92: 315–322.
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009; 113: 1875–1891.
Chendamarai E, Balasubramanian P, George B, Viswabandya A, Abraham A, Ahmed R et al. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood 2012; 119: 3413–3419.
Platzbecker U, Avvisati G, Ehninger G, Cicconi L, Thiede C, Ferrara F et al. Improved outcome with ATRA-arsenic trioxide compared to ATRA-chemotherapy in non-high risk acute promyelocytic leukemia – updated results of the Italian-German APL0406 Trial on the Extended Final Series. Blood 2014; 124: 12.
Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M et al. All-trans retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012; 120: 1570–1580.
Poiré X, Moser BK, Gallagher RE, Laumann K, Bloomfield CD, Powell BL et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma 2014; 55: 1523–1532.
Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood 2005; 106: 3768–3776.
Chillon MC, Santamaria C, Garcia-Sanz R, Balanzategui A, Sarasquete ME, Alcoceba M et al. Long FLT3 internal tandem duplications and reduced PML-RARα expression at diagnosis characterize a high-risk subgroup of acute promyelocytic leukemia patients. Haematologica 2010; 95: 745–751.
Schnittger S, Bacher U, Haferlach C, Kern W, Alpermann T, Haferlach T . Clinical impact of FLT3 mutation load in acute promyelocytic leukemia with t(15;17)/PML-RARA. Haematologica 2011; 96: 1799–1807.
Breccia M, Loglisci G, Loglisci MG, Ricci R, Diverio D, Latagliata R et al. FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica 2013; 98: e161–e163.
Molica M, Breccia M . FLT3-ITD in acute promyelocytic leukemia: clinical distinct profile but still controversial prognosis. Leuk Res 2015; 39: 397–399.
Acknowledgements
We thank all participants and research staff of all centers within GIMEMA. This study was supported by grants from Associazione Italiana contro le Leucemie (AIL) and Associazione Italiana per la Ricerca sul Cancro (AIRC, IG 5916, to Dr Lo-Coco).
Author contributions
LC, MD and FL-C. designed the study and wrote the paper; MD, CC, AF TO, LC, SL and VA performed the experiments; MD, FL-C, LC, MTV, FP, AL, SS and AP analysed data; MV, PF, GA, FF, PF, EDB, GS, MB, EC, MS, MGK, AS, SA, MTV and FM revised the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FL-C received honoraria from Lundbeck and Teva. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Cicconi, L., Divona, M., Ciardi, C. et al. PML–RARα kinetics and impact of FLT3–ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia 30, 1987–1992 (2016). https://doi.org/10.1038/leu.2016.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.122
This article is cited by
-
Critical transition and reversion of tumorigenesis
Experimental & Molecular Medicine (2023)
-
Significance of Dysplastic Neutrophils with Multiple Auer Rods in Post-therapy Acute Promyelocytic Leukemia
Indian Journal of Hematology and Blood Transfusion (2022)
-
All-trans Retinoic Acid, Arsenic Trioxide, and Anthracycline-based Chemotherapy Improves Outcome in Newly Diagnosed Acute Promyelocytic Leukemia Regardless of FLT3-ITD Mutation Status
Current Medical Science (2021)
-
Outcome of older (≥70 years) APL patients frontline treated with or without arsenic trioxide—an International Collaborative Study
Leukemia (2020)
-
NTAL is associated with treatment outcome, cell proliferation and differentiation in acute promyelocytic leukemia
Scientific Reports (2020)