Abstract
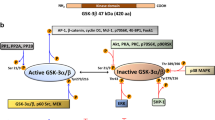
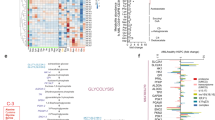
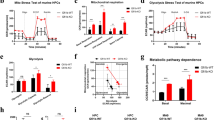
The rapid proliferation of myeloid leukemia cells is highly dependent on increased glucose metabolism. Through an unbiased metabolomics analysis of leukemia cells, we found that the glycogenic precursor UDP-D-glucose is pervasively upregulated, despite low glycogen levels. Targeting the rate-limiting glycogen synthase 1 (GYS1) not only decreased glycolytic flux but also increased activation of the glycogen-responsive AMP kinase (AMPK), leading to significant growth suppression. Further, genetic and pharmacological hyper-activation of AMPK was sufficient to induce the changes observed with GYS1 targeting. Cancer genomics data also indicate that elevated levels of the glycogenic enzymes GYS1/2 or GBE1 (glycogen branching enzyme 1) are associated with poor survival in AML. These results suggest a novel mechanism whereby leukemic cells sustain aberrant proliferation by suppressing excess AMPK activity through elevated glycogenic flux and provide a therapeutic entry point for targeting leukemia cell metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol 2014; 32: 415–423.
Lunt SY, Vander Heiden MG . Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27: 441–464.
Schulze A, Harris AL . How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012; 491: 364–373.
Warburg O . On respiratory impairment in cancer cells. Science 1956; 124: 269–270.
Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood 2005; 105: 1717–1723.
Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M . NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia 2011; 25: 281–289.
Rodrigues MS, Reddy MM, Sattler M . Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal 2008; 10: 1813–1848.
Reddy MM, Fernandes MS, Deshpande A, Weisberg E, Inguilizian HV, Abdel-Wahab O et al. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia 2012; 26: 481–489.
Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab 2012; 16: 751–764.
Pelletier J, Bellot G, Gounon P, Lacas-Gervais S, Pouyssegur J, Mazure NM . Glycogen synthesis is induced in hypoxia by the hypoxia-inducible factor and promotes cancer cell survival. Front Oncol 2012; 2: 18.
Seitz JF, Luganova IS . The biochemical identification of blood and bone marrow cells of patients with acute leukemia. Cancer Res 1968; 28: 2548–2555.
Valentine WN, Follette JH, Lawrence JS . The glycogen content of human leukocytes in health and in various disease states. J Clin Invest 1953; 32: 251–257.
Wagner R . Studies on the physiology of the white blood cell; the glycogen content of leukocytes in leukemia and polycythemia. Blood 1947; 2: 235–243.
Browner MF, Nakano K, Bang AG, Fletterick RJ . Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc Natl Acad Sci USA 1989; 86: 1443–1447.
Camici M, Ahmad Z, DePaoli-Roach AA, Roach PJ . Phosphorylation of rabbit liver glycogen synthase by multiple protein kinases. J Biol Chem 1984; 259: 2466–2473.
Nuttall FQ, Gannon MC, Bai G, Lee EY . Primary structure of human liver glycogen synthase deduced by cDNA cloning. Arch Biochem Biophys 1994; 311: 443–449.
Parker GE, Pederson BA, Obayashi M, Schroeder JM, Harris RA, Roach PJ . Gene expression profiling of mice with genetically modified muscle glycogen content. Biochem J 2006; 395: 137–145.
Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA et al. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab 2010; 12: 456–466.
Pederson BA, Wilson WA, Roach PJ . Glycogen synthase sensitivity to glucose-6-P is important for controlling glycogen accumulation in Saccharomyces cerevisiae. J Biol Chem 2004; 279: 13764–13768.
Preller A, Wilson CA, Quiroga-Roger D, Ureta T . Hexokinase and not glycogen synthase controls the flux through the glycogen synthesis pathway in frog oocytes. FEBS Lett 2013; 587: 2825–2831.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789.
Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P . Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem 1980; 107: 529–537.
Wang Y, Roach PJ . Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a). J Biol Chem 1993; 268: 23876–23880.
Embi N, Rylatt DB, Cohen P . Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 1980; 107: 519–527.
Woodgett JR . Judging a protein by more than its name: GSK-3. Sci STKE 2001; 2001: re12.
Carling D, Hardie DG . The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta 1989; 1012: 81–86.
Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F et al. The alpha2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 2004; 53: 3074–3081.
Burkewitz K, Zhang Y, Mair WB . AMPK at the Nexus of energetics and aging. Cell Metab 2014; 20: 10–25.
Hardie DG . AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8: 774–785.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003; 2: 28.
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996; 271: 27879–27887.
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 2004; 101: 3329–3335.
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003; 13: 2004–2008.
Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011; 472: 230–233.
Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res 2002; 62: 3659–3662.
Yuan M, Breitkopf SB, Yang X, Asara JM . A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012; 7: 872–881.
Ferrer JC, Favre C, Gomis RR, Fernandez-Novell JM, Garcia-Rocha M, de la Iglesia N et al. Control of glycogen deposition. FEBS Lett 2003; 546: 127–132.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Sutherland C, Cohen P . The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett 1994; 338: 37–42.
Sutherland C, Leighton IA, Cohen P . Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 1993; 296: 15–19.
Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci USA 2011; 108: 528–533.
Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 2006; 3: 403–416.
Corton JM, Gillespie JG, Hawley SA, Hardie DG . 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 1995; 229: 558–565.
Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 2003; 102: 987–995.
Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood 2010; 116: 4262–4273.
Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M et al. Pterostilbene and 3'-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol 2005; 37: 1709–1726.
Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC . Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood 2011; 118: 6399–6402.
Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA . Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem 1998; 273: 35347–35354.
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 2013; 17: 113–124.
Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C . BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood 2002; 100: 3767–3775.
Rodriguez-Enriquez S, Torres-Marquez ME, Moreno-Sanchez R . Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch Biochem Biophys 2000; 375: 21–30.
Dworkin MB, Dworkin-Rastl E . Metabolic regulation during early frog development: glycogenic flux in Xenopus oocytes, eggs, and embryos. Dev Biol 1989; 132: 512–523.
Rousset M, Zweibaum A, Fogh J . Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res 1981; 41: 1165–1170.
Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW et al. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell 2010; 143: 711–724.
McBride A, Ghilagaber S, Nikolaev A, Hardie DG . The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab 2009; 9: 23–34.
Melendez-Hevia E, Waddell TG, Shelton ED . Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem J 1993; 295: 477–483.
Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL et al. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem 2005; 280: 17260–17265.
Pederson BA, Schroeder JM, Parker GE, Smith MW, DePaoli-Roach AA, Roach PJ . Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes 2005; 54: 3466–3473.
Irimia JM, Meyer CM, Peper CL, Zhai L, Bock CB, Previs SF et al. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J Biol Chem 2010; 285: 12851–12861.
Lee YC, Chang CJ, Bali D, Chen YT, Yan YT . Glycogen-branching enzyme deficiency leads to abnormal cardiac development: novel insights into glycogen storage disease IV. Hum Mol Genet 2011; 20: 455–465.
Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 2007; 26: 1616–1625.
Acknowledgements
This work is supported in part by National Institutes of Health grant CA134660 (MS), CA6696 (JDG) and by the Gloria Spivak Faculty Advancement Fund (MS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Bhanot, H., Reddy, M., Nonami, A. et al. Pathological glycogenesis through glycogen synthase 1 and suppression of excessive AMP kinase activity in myeloid leukemia cells. Leukemia 29, 1555–1563 (2015). https://doi.org/10.1038/leu.2015.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.46
This article is cited by
-
Metabolic targeting of malignant tumors: a need for systemic approach
Journal of Cancer Research and Clinical Oncology (2023)
-
Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma
Nature Metabolism (2021)
-
Integrated genomic-metabolic classification of acute myeloid leukemia defines a subgroup with NPM1 and cohesin/DNA damage mutations
Leukemia (2021)
-
Hypoxia-induced GBE1 expression promotes tumor progression through metabolic reprogramming in lung adenocarcinoma
Signal Transduction and Targeted Therapy (2020)
-
The differential activation of metabolic pathways in leukemic cells depending on their genotype and micro-environmental stress
Metabolomics (2020)