Abstract
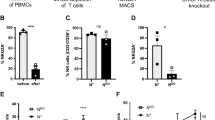
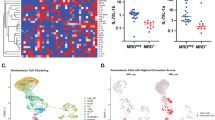
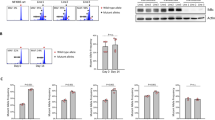
Natural killer (NK) cells are key components of the innate immune system, providing potent antitumor immunity. Here, we show that the tumor growth factor-β (TGF-β)/SMAD signaling pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia (ALL). We characterized NK cells in 50 consecutive children with B-ALL at diagnosis, end induction and during maintenance therapy compared with age-matched controls. ALL-NK cells at diagnosis had an inhibitory phenotype associated with impaired function, most notably interferon-γ production and cytotoxicity. By maintenance therapy, these phenotypic and functional abnormalities partially normalized; however, cytotoxicity against autologous blasts remained impaired. We identified ALL-derived TGF-β1 to be an important mediator of leukemia-induced NK cell dysfunction. The TGF-β/SMAD signaling pathway was constitutively activated in ALL-NK cells at diagnosis and end induction when compared with healthy controls and patients during maintenance therapy. Culture of ALL blasts with healthy NK cells induced NK dysfunction and an inhibitory phenotype, mediated by activation of the TGF-β/SMAD signaling pathway, and abrogated by blocking TGF-β. These data indicate that by regulating the TGF-β/SMAD pathway, ALL blasts induce changes in NK cells to evade innate immune surveillance, thus highlighting the importance of developing novel therapies to target this inhibitory pathway and restore antileukemic cytotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 2012; 30: 1663–1669.
Lotzova E, Savary CA, Herberman RB . Inhibition of clonogenic growth of fresh leukemia cells by unstimulated and IL-2 stimulated NK cells of normal donors. Leuk Res 1987; 11: 1059–1066.
Lowdell MW, Craston R, Samuel D, Wood ME, O'Neill E, Saha V et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol 2002; 117: 821–827.
Lanier LL . Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9: 495–502.
Ljunggren HG, Karre K . In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today 1990; 11: 237–244.
Marsh SG, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D et al. Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Tissue Antigens 2003; 62: 79–86.
Molfetta R, Quatrini L, Capuano C, Gasparrini F, Zitti B, Zingoni A et al. c-Cbl regulates. Eur J Immunol 2014; 44: 2761–2770.
Karre K . Immunology. A perfect mismatch. Science 2002; 295: 2029–2031.
Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L . Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 2014; 44: 1582–1592.
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ . Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29: 235–271.
Whiteside TL . The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27: 5904–5912.
Zitvogel L, Tesniere A, Kroemer G . Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6: 715–727.
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99: 836–847.
Trotta R, Dal CJ, Yu J, Ciarlariello D, Thomas B, Zhang X et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol 2008; 181: 3784–3792.
Alter G, Malenfant JM, Altfeld M . CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22.
Weber G, Caruana I, Rouce RH, Barrett AJ, Gerdemann U, Leen AM et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia—implications for immunotherapy. Clin Cancer Res 2013; 19: 5079–5091.
Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012; 14: 1131–1143.
Hassett J, Parker J . Laboratory practices in reporting flow cytometry phenotyping results for leukemia/lymphoma specimens: results of a survey. Cytometry 1995; 22: 264–281.
Brando B, Sommaruga E . Nationwide quality control trial on lymphocyte immunophenotyping and flow cytometer performance in Italy. Cytometry 1993; 14: 294–306.
Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol 2010; 40: 3347–3357.
Jewett A, Tseng HC . Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer 2011; 2: 443–457.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71: 5393–5399.
Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 2013; 190: 5321–5328.
Lee HM, Kim KS, Kim J . A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta. Cell Immunol 2014; 290: 52–61.
Yang B, Liu H, Shi W, Wang Z, Sun S, Zhang G et al. Blocking transforming growth factor-beta signaling pathway augments antitumor effect of adoptive NK-92 cell therapy. Int Immunopharmacol 2013; 17: 198–204.
Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 2002; 99: 3661–3667.
Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007; 109: 4816–4824.
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121: 3609–3622.
Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ . Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med 1994; 180: 1427–1435.
Bryceson YT, March ME, Ljunggren HG, Long EO . Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107: 159–166.
North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol 2007; 178: 85–94.
Torelli GF, Peragine N, Raponi S, Pagliara D, De Propris MS, Vitale A et al. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica 2014; 99: 1248–1254.
Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014; 124: 2034–2045.
DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013; 27: 170–182.
Mauri C, Blair PA . The incognito journey of a regulatory B cell. Immunity 2014; 41: 878–880.
Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood 1996; 87: 1928–1938.
Shehata M, Schwarzmeier JD, Hilgarth M, Hubmann R, Duechler M, Gisslinger H . TGF-beta1 induces bone marrow reticulin fibrosis in hairy cell leukemia. J Clin Invest 2004; 113: 676–685.
Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U . Regulation of NK cell functions by TGF-beta 1. J Immunol 1995; 155: 1066–1073.
Laouar Y, Sutterwala FS, Gorelik L, Flavell RA . Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 2005; 6: 600–607.
Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 2002; 99: 3179–3187.
Dennler S, Pendaries V, Tacheau C, Costas MA, Mauviel A, Verrecchia F . The steroid receptor co-activator-1 (SRC-1) potentiates TGF-beta/Smad signaling: role of p300/CBP. Oncogene 2005; 24: 1936–1945.
Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 2012; 11: 2674–2684.
Shimasaki N, Campana D . Natural killer cell reprogramming with chimeric immune receptors. Methods Mol Biol 2013; 969: 203–220.
Jardine L, Hambleton S, Bigley V, Pagan S, Wang XN, Collin M . Sensitizing primary acute lymphoblastic leukemia to natural killer cell recognition by induction of NKG2D ligands. Leuk Lymphoma 2013; 54: 167–173.
Acknowledgements
We are thank the patients and their families for their cooperation. We are grateful to Amos Gaikwad and Tatiana Goltsova for flow cytometry assistance; Drs Cliona M Rooney and Natalia Lapteva for providing critical experimental components. This research was partially performed in the Flow Cytometry and Cellular Imaging Facility at MD Anderson Cancer Center, which is supported, in part, by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672. This research was funded, in part, by the MD Anderson Chronic Lymphocytic Leukemia Moon Shot Program. We also appreciate the support of shared resources by Dan L Duncan Cancer Center support grant P30CA125123. LLS SCOR (to CMB, KRR), Children’s Leukemia Research Association (to CMB, KRR), St Baldrick Scholar Award (to KRR), MDACC Leukemia SPORE Grant CA 100632 and SINF (to KR), Lymphoma SPORE P50CA126752 (to RHR) and NIH Training Grant T32 HL092332 (to RHR).
Author contributions
RHR designed research, performed research, analyzed data and wrote the paper. HS designed research, performed research and analyzed data. TS performed research and analyzed data. GW performed research. SK performed research. BB performed research. CB performed research. VM performed research. ES analyzed data. CMB designed research, analyzed data and wrote the paper. KRR designed research, analyzed data and wrote the paper. KR designed research, analyzed data and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Rouce, R., Shaim, H., Sekine, T. et al. The TGF-β/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 30, 800–811 (2016). https://doi.org/10.1038/leu.2015.327
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.327
This article is cited by
-
Th1 cytokines in pediatric acute lymphoblastic leukemia
Cancer Immunology, Immunotherapy (2023)
-
Circular EZH2-encoded EZH2-92aa mediates immune evasion in glioblastoma via inhibition of surface NKG2D ligands
Nature Communications (2022)
-
Prolactin Acts on Myeloid Progenitors to Modulate SMAD7 Expression and Enhance Hematopoietic Stem Cell Differentiation into the NK Cell Lineage
Scientific Reports (2020)
-
Advances in targeted therapy for malignant lymphoma
Signal Transduction and Targeted Therapy (2020)
-
Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition
Nature Communications (2020)