Abstract
Renal insufficiency (RI) is a frequent complication of multiple myeloma (MM) with negative consequences for patient survival. The improved clinical outcome with successive Total Therapy (TT) protocols was limited to patients without RI. We therefore performed a retrospective analysis of overall survival, progression-free survival and time to progression (TTP) of patients enrolled in TT2 and TT3 in relationship to RI present at baseline and pre-transplant. Glomerular filtration rate was graded in four renal classes (RCs), RC1–RC4 (RC1 ⩾90 ml/min/1.73 m2, RC2 60–89 ml/min/1.73 m2, RC3 30–59 ml/min/1.73 m2 and RC4 <30 ml/min/1.73 m2). RC1–3 had comparable clinical outcomes while RC4 was deleterious, even after improvement to better RC after transplant. Among the 85% of patients with gene expression profiling defined low-risk MM, Cox regression modeling of baseline and pre-transplant features, which also took into consideration RC improvement and MM complete response (CR), identified the presence of metaphase cytogenetic abnormalities and baseline RC4 as independent variables linked to inferior TTP post-transplant, while MM CR reduced the risk of progression and TTP by more than 60%. Failure to improve clinical outcomes despite RI improvement suggested MM-related causes. Although distinguishing RC4 from RC<4, 46 gene probes bore no apparent relationship to MM biology or survival.
Similar content being viewed by others
Introduction
Renal insufficiency (RI) is a common complication of multiple myeloma (MM) that can be present at diagnosis or emerge during therapy1, 2 and represents a feature of Cancer Research and Biostatistics criteria constituting the need for instituting MM therapy.3 The etiology of RI is often multifactorial; hypercalcemia and any of the myeloma-protein-associated conditions such as light-chain cast nephropathy, light-chain amyloidosis and light-chain deposition disease are common causes. Hypercalcemia and light-chain cast nephropathy readily respond to hydration and effective myeloma therapy.4, 5, 6 In case of high tumor burden and high-grade characteristics, effective treatment can provoke tumor lysis and thus cause renal shut-down.7 Additionally, nephrotoxic antibiotics and bisphosphonates can aggravate or cause renal impairment. The intricate interplay between renal function and MM is complex and of interest because RI has important implications for survival. The adverse survival consequences of RI have long been acknowledged,2, 8 accounting for the B sub-stage designation in the Durie-Salmon staging system.9 RI as a prognosticator for survival has been retained indirectly in the albumin- and β-2-microglobulin (B2M)-based International Staging System.10 The B2M molecule is shed from the surface of MM cells so that its serum levels reflect tumor burden,11 but due to the renal excretion of B2M, RI can further raise B2M serum levels.12 Poor clinical outcomes resulting from RI are usually attributed to higher treatment-related mortality.12, 13, 14, 15, 16, 17
The introduction of bortezomib has greatly improved survival outcomes in MM, both in transplant and non-transplant settings. When bortezomib, not requiring adjustment for renal function, was added to melphalan-prednisone in the VISTA trial, RI was not an adverse feature in the experimental arm.18 In the HOVON-65/GMMG-HD4 trial, the prognostic impact of RI was investigated in two treatment arms, both including melphalan-based auto-transplants.13 One arm received bortezomib, adriamycin and dexamethasone (PAD) induction prior to and bortezomib maintenance after autologous stem cell transplant, while the other arm was given vincristine, adriamycin and dexamethasone (VAD) induction and thalidomide maintenance. Although renal response rates were similar after PAD and VAD, MM response rates including complete response were higher with PAD, as were overall survival (OS) and progression-free survival (PFS). In fact, OS and PFS were independent of RI in the PAD arm and resembled outcomes of patients without RI treated with VAD. The observation of similar renal responses to VAD and PAD and yet inferior survival with VAD suggested a RI-associated adverse MM feature that could be overcome by the inclusion of bortezomib in PAD.
Reviewing clinical outcomes after successive Total Therapy (TT) trials, significant survival advances were observed with the transition from TT1 to TT2 and TT3.19 The incorporation of bortezomib into induction, consolidation and maintenance phases of TT3 led to dramatic improvement in clinical outcomes that, however, was limited to the 85% of patients with plasma cell gene expression profiling (GEP)-defined low risk. The 15% with high-risk disease (GEP70>0.66) continued to fare poorly despite the addition of bortezomib and immune-modulatory agents. We also observed that there was a lack of progress in the transition from TT2 to TT3 in the case of RI. We now examine whether the lack of clinical outcome improvement was limited to patients who did not improve from baseline RI.
Materials and methods
Records were reviewed from our data base of all MM patients (N=1148) enrolled and followed in TT2 without thalidomide (TT2−Thal; n=345), TT2 with thalidomide (TT2+Thal; n=323), and TT3 (n=480) between 14 October 1998 and 29 January 2014. Details of these protocols and patient outcomes have previously been reported.20, 21, 22, 23 TT3b (n=177) differed from TT3a (n=303) only in the maintenance phase such that in TT3b bortezomib, lenalidomide and dexamethasone was applied for all 3 years whereas TT3a employed bortezomib, thalidomide and dexamethasone only in the first year and subsequently only thalidomide and dexamethasone. In TT2, RI was not an exclusion criterion as long as it was of recent onset (<2 months) and due to Bence Jones proteinuria or hypercalcemia. Cisplatin dosing in cycle 2 with dexamethasone, cyclophosphamide, etoposide and cisplatin was modified according to severity of RI. Patients with serum creatinine ⩽1.5 mg/dl received 15 mg/m2; cisplatin dosing was reduced to 10 mg/m2 for creatinine of 1.6–2.0 mg/m2 and to 7.5 mg/m2 for creatinine 2.1–3.0 mg/dl, while it was omitted in case of creatinine >3.0 mg/m2. TT2 also restricted melphalan dosing to 140 mg/m2 during the transplant phase when serum creatinine was ⩾3.0 mg/dl. TT3 patients were eligible when serum creatinine values did not exceed 3 mg/dl. Induction cisplatin was modified from the full dose of 10 mg/m2 to 5 mg/m2 for serum creatinine of 1.6–2 mg/dl and drug was omitted with creatinine >2 mg/dl. As in TT2, melphalan dosing in the transplant phase was reduced to 140 mg/m2 for creatinine levels of ⩾3.0 mg/dl. GEP risk designation was applied as previously reported.24
Clinical outcome data included OS and PFS. We also examined time to progression (TTP). For the purpose of this analysis, we calculated the estimated glomerular filtration rate (eGFR) for all patients, using the original Modification of Diet in Renal Disease equation25 as recommended by International Myeloma Working Group.5 The aforementioned clinical end points were examined among four renal classes (RCs): RC1 (eGFR ⩾90 ml/min/1.73 m2), RC2 (eGFR 60–89 ml/min/1.73 m2), RC3 (eGFR 30–59 ml/min/1.73 m2) and RC4 (eGFR <30 ml/min/1.73 m2).
All protocols had been approved by the University of Arkansas Medical Sciences Institutional Review Board. Patients were required to sign a written informed consent in keeping with institutional, federal and the Declaration of Helsinki guidelines. Annual Data Safety and Monitoring Board and semi-annual external auditor reviews were also performed according to National Institutes of Health mandates for federally supported research grants.
Statistical analyses
Univariate baseline characteristics were compared between protocols using χ2-tests. RC comparisons were made between RC1-3 and RC4, since OS and PFS were similar between RC1, RC2 and RC3, while RC4 predicted uniquely poor OS and PFS. Kaplan–Meier curves were compared using the log-rank test.26 TTP was analyzed by estimating the cumulative incidence of the given outcome,27 and compared between RC groups (1–3 vs 4) by the log-rank test. Cox proportional hazards regression models were employed to identify associations between RC groups (1–3 vs 4) and survival outcomes.28 Multivariate models were arrived at using stepwise model selection with entry level P-value of 0.1, where the variable remained in the model if it was significant at the 0.05 level. An indicator of TT3 protocol was included in the multivariate analyses to account for the use of bortezomib in TT3a and TT3b compared with TT2 regimens. Employing a false discovery rate of q=0.05, 46 significant gene probes were identified that distinguished these RI subsets.29
Results
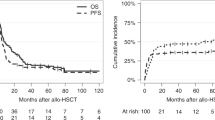
Patients' baseline characteristics including RC distributions were largely similar across the treatment regimens. TT3 comprised higher proportions of patients with high B2M levels >5.5 mg/l, hypo-albuminemia <3.5 g/dl and Bence Jones proteinuria (Table 1). OS and PFS are depicted across all protocols according to the baseline RC (Figure 1). Clinical outcomes in patients with RC1–3 bundled together, while RC4 was associated with inferior OS (Figure 1a) and PFS (Figure 1b). Given the strikingly different outcomes between patients in RC1–3 versus RC4 classes, we next examined the outcomes in these two RC groups by protocol (Figure 2). Both OS and PFS improved markedly with the transition from TT2−Thal to TT2+Thal to TT3 in patients with RC1–3 (Figures 2a and c), however, such progress was not apparent in RC4 (Figures 2b and d). One possible explanation might be that RC4 patients could not be treated in as timely a fashion as their RC1–3 counterparts. However, the succession through protocol phases was similar in RC4 and RC1–3 groups across protocols (data not shown). Examining PFS in order to capture both progressions and deaths, we employed Cox-regression analysis with all relevant baseline variables. The univariately adverse effect of RC4 (hazards ratio=1.73, P<0.001) was not retained after adjustment for other parameters, whether or not GEP70 risk was considered (Tables 2A and 2B). In fact, well-recognized standard features like low albumin, elevated serum levels of B2M and lactate dehydrogenase, cytogenetic abnormalities (CA), low platelet count and immunoglobulin A isotype all imparted shorter PFS, joined by GEP70 high risk when this variable was included (Table 2B); TT3 reduced the hazard of progression by about 40%. When limited to GEP70 low-risk MM, RC4 remained a strong adverse feature after adjusting for the other parameters (Table 2C).
Subsequent analyses concentrated on the GEP70 low-risk cohort, in order to focus on the population for which RC4 imparts poorer outcomes relative to RC1–3. To emphasize MM-related events, we investigated TTP in GEP70-defined low-risk MM (Figure 3). The significantly steeper onset of progression in RC4 compared with RC1–3 patients suggests that the difference in survival is MM related. This observation prompted us to investigate whether, in patients with GEP70 low-risk MM, there were RC-dependent differences in baseline characteristics. Indeed, although the frequency of CA overall was similar between RC groups (41% with RC4 vs 31% with RC1–3; P=0.07), high-risk CA types especially CA13 and/or hypodiploidy were overrepresented in RC4 (40% vs 19% in RC<4; P<0.001). Logistic regression analysis that excluded creatinine and B2M for their known correlations with eGFR revealed that high C-reactive protein (⩾8 mg/l, hemoglobin <10 g/dl, high lactate dehydrogenase (⩾190 U/l) and bone marrow plasmacytosis (⩾33%) were all independently linked to RC4 (Table 3).
Then we examined GEP signatures among GEP70-defined low-risk MM relative to RC4 and RC<4 groups. Employing a false discovery rate of q=0.05, 46 significant gene probes were identified that distinguished these RI subsets (Supplementary Tables 1A and 1B). We failed to recognize a plausible relationship of the listed gene probes to MM biology, and an impact on survival was not apparent.
In the following section, we analyze the effect of RC improvement after induction therapy on post-transplant outcomes in low-risk MM (Figure 4). Both OS and PFS were superior when RC1–3 status was maintained pre-transplant (Figures 4a and b). The gravest outcome applied to patients who moved from RC1–3 at baseline to RC4 at transplant, although there are very few patients in this group. The remaining patients (RC4 at both time points, RC4 improving to RC1–3) had an intermediate outcome. The same directional effect applied to TTP (Figure 4c). Next, we investigated whether our GEP46 model could distinguish, at baseline, the RC4 to RC1–3 converts from patients retaining RC4 status. Results revealed, possibly due to small sample size, no baseline differences between these two groups (data not shown).
Overall survival, progression-free survival and time to progression in GEP70-defined low-risk myeloma according to baseline and transplant RCs. (a) Overall survival is superior with RC1–3 at both time points; (b) progression-free survival is superior with RC1–3 at both time points; (c) time to progression most shallow with RC1–3 at both time points.
In order to account for baseline and pre-transplant variables collectively in the context of RC change and clinical response, a further multivariate analysis examining PFS and TTP was performed among the GEP70-defined low-risk patients (Table 4). Unfortunately, small sample sizes in the baseline RC4 group hindered this analysis. We do note a significant increase in the hazard for progression among patients with baseline RC4 who improved to RC1–3 at transplant compared with patients with RC1–3 at both time points, showing that even when patients recover from RI before transplant, they still have worse outcome compared with patients with baseline RC1–3. There were trends for increased hazard of outcome (both PFS and TTP) for the baseline RC4 groups (with either RC1–3 or RC4 at the time of transplant) compared with the group with RC1–3 at both baseline and transplant. Of interest, among the subset of patients with improvement from RC4 to RC1–3 before transplant, the majority did not revert back to RC4 upon disease relapse (data not shown). The models were dominated by the well-recognized baseline prognostic variables (albumin, B2M, C-reactive protein, platelet count, IgA isotype and CA). The inclusion of bortezomib in TT3a and TT3b improved both PFS and TTP when other variables were accounted for. Achieving MM complete response status before transplant was the only beneficial post-treatment variable.
Discussion
We validated the adverse prognostic consequences of RI as measured by eGFR values <30 ml/min/1.73 m2. The improvement in clinical outcomes over the course of TT2 and TT3 protocols was limited to patients lacking RC4. The failure of RC4 patients to derive benefit from bortezomib in TT3 trials is at variance with HOVON data.13 Baseline RC4 maintained its adverse impact in multivariate analyses among the subset of GEP70-defined low-risk patients, even if RC1–3 was achieved by the time of transplant. Based on the TTP analyses performed, this adverse impact appears to be MM-related, which is supported also by overrepresentation of prognostically adverse MM features (anemia; elevated levels of lactate dehydrogenase, C-reactive protein and bone marrow plasma cells; and presence of CA13 and/or hypodiploidy). Pursuing a MM-related RC4 prognostic feature, we identified 46 gene probes distinguished RC4 from RC1–3 classes which, however, did not appear to relate to MM biology or survival.
References
Heher EC, Rennke HG, Laubach JP, Richardson PG . Kidney disease and multiple myeloma. Clin J Am Soc Nephrol 2013; 8: 2007–2017.
Alexanian R, Barlogie B, Dixon D . Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med 1990; 150: 1693–1695.
Kyle RA, Rajkumar SV . Crietria for diagnosis, staging and risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H . Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008; 22: 1485–1493.
Leung N, Dispenzieri A, Lacy MQ, Kumar SK, Hayman SR, Fervenza FC et al. Severity of baseline proteinuria predicts renal response in immunoglobulin light chain-associated amyloidosis after autologous stem cell transplantation. Clin J Am Soc Nephrol 2007; 2: 440–444.
Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 2010; 28: 4976–4984.
Huston A, Brown J, Roodman GD . Tumor lysis syndrome following thalidomide and dexamethasone therapy for newly diagnosed multiple myeloma. Exp Hematol 2006; 34: 1616.
Knudsen LM, Hjorth M, Hippe E . Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000; 65: 175–181.
Durie BG, Salmon SE . A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975; 36: 842–854.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Perosa F, Minoia C, Favoino E, Prete M, Dammacco F . Staging multiple myeloma patients with active disease using serum levels of beta2m-free HLA class I heavy chain together with IgM or platelet count. Blood Cells Mol Dis 2009; 42: 71–76.
Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol 2001; 114: 822–829.
Scheid C, Sonneveld P, Schmidt-Wolf IG, van der Holt B, el Jarari L, Bertsch U et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica 2014; 99: 148–154.
Bird JM, Fuge R, Sirohi B, Apperley JF, Hunter A, Snowden J et al. The clinical outcome and toxicity of high-dose chemotherapy and autologous stem cell transplantation in patients with myeloma or amyloid and severe renal impairment: a British Society of Blood and Marrow Transplantation study. Br J Haematol 2006; 134: 385–390, 20.
Knudsen LM, Nielsen B, Gimsing P, Geisler C . Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. Eur J Haematol 2005; 75: 27–33.
Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 2007; 48: 337–341.
Blade J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998; 158: 1889–1893.
Jesus F, Miguel S, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med 2008; 359: 906–917.
Usmani SZ, Crowley J, Hoering A, Mitchell A, Waheed S, Nair B et al. Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured? Leukemia 2013; 27: 226–232.
Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med 2006; 354: 1021–1030.
Van Rhee F, Szymonifka J, Anaissie E, Nair B, Waheed S, Alsayed Y et al. Total Therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood 2010; 116: 1220–1227.
Nair B, van Rhee F, Shaughnessy Jr JD, Anaissie E, Szymonifka J, Hoering A et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling–defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood 2010; 115: 4168–4173.
Barlogie B, Mitchell A, van Rhee F, Epstein J, Morgan G, Crowley J . Curing myeloma at last-defining criteria and providing the evidence. Blood 2014; 124: 3043–3051.
Shaughnessy Jr JD, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2007; 109: 2276–2284.
Kooman JP . Estimation of renal function in patients with chronic kidney disease. J Magn Reson Imaging 2009; 30: 1341–1346.
Mantel N . Evaluation or survival data and two new rank order statistics srising in ots evaluation. Cancer Chemo Rep 1966; 50: 163–170.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Cox DR . Regression models and life-tables (with discussion). J Royal Statist Soc Series B 1972; 34: 187–220.
Storey JD, Tibshirani R . Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003; 100: 9440–9445.
Acknowledgements
This work was supported by a grant from the National Cancer Institute, National Institutes of Health (grant number CA 55813).
Author Contributions
RK, SA, MG and BB conceptualized and wrote the paper. MG, SA, FvR, SW, SU, SA, SK and BB treated patients and participated in clinical research protocols. AR, AH and JC analyzed data and performed statistical analyses. SY, JE, and JDS performed genomic interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Barlogie has received research funding from Celgene and Millennium, is a consultant to Celgene and Millennium, and is a co-inventor on patents and patent applications related to use of GEP in cancer medicine, that have been licensed to Myeloma Health, LLC.
Additional information
Supplementary Information accompanies this paper on the Leukemia website .
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Khan, R., Apewokin, S., Grazziutti, M. et al. Renal insufficiency retains adverse prognostic implications despite renal function improvement following Total Therapy for newly diagnosed multiple myeloma. Leukemia 29, 1195–1201 (2015). https://doi.org/10.1038/leu.2015.15
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.15
This article is cited by
-
Acute myeloma kidney and SARS-COV2 infection with dialysis need: never say never - a case report
BMC Nephrology (2023)
-
Autologous stem cell transplantation in multiple myeloma patients with renal impairment
Annals of Hematology (2023)
-
Genomic characterization of functional high-risk multiple myeloma patients
Blood Cancer Journal (2022)
-
Association of serum calcium levels with renal impairment and all-cause death in Chinese patients with newly diagnosed multiple myeloma: a cross-sectional, longitudinal study
Nutrition & Metabolism (2021)
-
Anti-BCMA CAR-T Cell Therapy in Relapsed or Refractory Multiple Myeloma Patients with Impaired Renal Function
Current Medical Science (2021)