Abstract
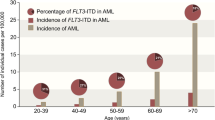
Acute myeloid leukemia (AML) is the second most common form of leukemia and the most frequent cause of leukemia-related deaths in the United States. The incidence of AML increases with advancing age and the prognosis for patients with AML worsens substantially with increasing age. Many older patients are ineligible for intensive treatment and require other therapeutic approaches to optimize clinical outcome. To address this treatment gap, novel agents with varying mechanisms of action targeting different cellular processes are currently in development. Hypomethylating agents (azacitidine, decitabine, SGI-110), histone deacetylase inhibitors (vorinostat, pracinostat, panobinostat), FMS-like tyrosine kinase receptor-3 inhibitors (quizartinib, sorafenib, midostaurin, crenolanib), cytotoxic agents (clofarabine, sapacitabine, vosaroxin), cell cycle inhibitors (barasertib, volasertib, rigosertib) and monoclonal antibodies (gentuzumab ozogamicin, lintuzumab-Ac225) represent some of these promising new treatments. This review provides an overview of novel agents that have either completed or are currently in ongoing phase III trials in patients with previously untreated AML for whom intensive treatment is not an option. Other potential drugs in earlier stages of development will also be addressed in this review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29.
Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD, based on November 2010 SEER data submission. 2011. Available at http://seer.cancer.gov/csr/1975_2008/.
Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074.
Bernasconi P . Molecular pathways in myelodysplastic syndromes and acute myeloid leukemia: relationships and distinctions-a review. Br J Haematol 2008; 142: 695–708.
Abdel-Wahab O, Levine RL . Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood 2013; 121: 3563–3572.
Tabe Y, Konopleva M . Advances in understanding the leukaemia microenvironment. Br J Haematol 2014; 164: 767–778.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J et alPrognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012; 366: 1079–1089.
Marcucci G, Haferlach T, Dohner H . Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol 2011; 29: 475–486.
Grossmann V, Schnittger S, Kohlmann A, Eder C, Roller A, Dicker F et alA novel hierarchical prognostic model of AML solely based on molecular mutations. Blood 2012; 120: 2963–2972.
Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B et alLong-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol 2011; 29: 2758–2765.
Kantarjian H, O'Brien S . Questions regarding frontline therapy of acute myeloid leukemia. Cancer 2010; 116: 4896–4901.
Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK et alA comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 2007; 109: 1114–1124.
Burnett AK, Russell NH, Hunter AE, Milligan D, Knapper S, Wheatley K et alClofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood 2013; 122: 1384–1394.
Harousseau JL, Martinelli G, Jedrzejczak WW, Brandwein JM, Bordessoule D, Masszi T et alA randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood 2009; 114: 1166–1173.
Issa JP . DNA methylation as a therapeutic target in cancer. Clin Cancer Res 2007; 13: 1634–1637.
Grövdal M, Khan R, Aggerholm A, Antunovic P, Astermark J, Bernell P et alNegative effect of DNA hypermethylation on the outcome of intensive chemotherapy in older patients with high-risk myelodysplastic syndromes and acute myeloid leukemia following myelodysplastic syndrome. Clin Cancer Res 2007; 13: 7107–7112.
Shen L, Kantarjian H, Guo Y, Lin E, Shan J, Huang X et alDNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol 2010; 28: 605–613.
Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N et alA comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One 2010; 5: e9001.
Schmelz K, Wagner M, Dorken B, Tamm I . 5-Aza-2'-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia. Int J Cancer 2005; 114: 683–695.
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Myelodysplastic SyndromeVersion 2. 2013. Available at http://www.nccn.org.
Saunthararajah Y, Triozzi P, Rini B, Singh A, Radivoyevitch T, Sekeres M et alp53-Independent, normal stem cell sparing epigenetic differentiation therapy for myeloid and other malignancies. Semin Oncol 2012; 39: 97–108.
Kayser S, Zucknick M, Dohner K, Krauter J, Kohne CH, Horst HA et alMonosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood 2012; 119: 551–558.
Bally C, Ades L, Renneville A, Sebert M, Eclache V, Preudhomme C et alPrognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk Res 2014; 38: 751–755.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et alEfficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U et alAzacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010; 28: 562–569.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH et alResults of a phase 3, multicenter, randomized, open-label study of azacitidine (AZA) vs conventional care regimens (CCR) in older patients with newly diagnosed acute myeloid leukemia (AML). Haematologica 2014; 99 (Suppl 1): 788–789, (abstract LB2433).
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J et alMulticenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670–2677.
Astex Pharmaceuticals IncPress release: FDA’s Oncologic Drugs Advisory Committee (ODAC) votes to not support benefit/risk profile of Dacogen® (decitabine) in acute myeloid leukemia. 9 February 2013. Available at http://investor.astx.com/releasedetail.cfm?ReleaseID=647666.
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Acute Myeloid Leukemia. Version 2. 2013. Available at http://www.nccn.org.
Leyden M, Manoharan A, Boyd A, Cheng ZM, Sullivan J . Low dose cytosine arabinoside: partial remission of acute myeloid leukaemia without evidence of differentiation induction. Br J Haematol 1984; 57: 301–307.
Housset M, Daniel MT, Degos L . Small doses of ARA-C in the treatment of acute myeloid leukaemia: differentiation of myeloid leukaemia cells? Br J Haematol 1982; 51: 125–129.
Bonate PL, Arthaud L, Cantrell Jr WR, Stephenson K, Secrist JA III, Weitman S . Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov 2006; 5: 855–863.
Kantarjian HM, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T et alPhase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol 2010; 28: 549–555.
Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z et alA randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2008; 112: 1638–1645.
Hanaoka K, Suzuki M, Kobayashi T, Tanzawa F, Tanaka K, Shibayama T et alAntitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-beta-D-arabino-pentofuranosyl) cytosine) and its N4-palmitoyl derivative (CS-682). Int J Cancer 1999; 82: 226–236.
Liu X, Guo Y, Li Y, Jiang Y, Chubb S, Azuma A et alMolecular basis for G2 arrest induced by 2'-C-cyano-2'-deoxy-1-beta-D-arabino-pentofuranosylcytosine and consequences of checkpoint abrogation. Cancer Res 2005; 65: 6874–6881.
Liu XJ, Nowak B, Wang YQ, Plunkett W . Sapacitabine, the prodrug of CNDAC, is a nucleoside analog with a unique action mechanism of inducing DNA strand breaks. Chin J Cancer 2012; 31: 373–380.
Kantarjian H, Faderl S, Garcia-Manero G, Luger S, Venugopal P, Maness L et alOral sapacitabine for the treatment of acute myeloid leukaemia in elderly patients: a randomised phase 2 study. Lancet Oncol 2012; 13: 1096–1104.
Ravandi F, Kadia TM, Borthakur G, Wierda WG, Goldberg SL, Wetzler M et alPooled analysis of elderly patients with newly diagnosed AML treated with sapacitabine and decitabine administered in alternating cycles. Blood 2012; 120: 2630.
Mortlock AA, Foote KM, Heron NM, Jung FH, Pasquet G, Lohmann JJ et alDiscovery, synthesis, and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J Med Chem 2007; 50: 2213–2224.
Mistry HB, MacCallum DE, Jackson RC, Chaplain MA, Davidson FA . Modeling the temporal evolution of the spindle assembly checkpoint and role of Aurora B kinase. Proc Natl Acad Sci USA 2008; 105: 20215–20220.
Ikezoe T, Yang J, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y et alA novel treatment strategy targeting Aurora kinases in acute myelogenous leukemia. Mol Cancer Ther 2007; 6: 1851–1857.
Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y et alAZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood 2007; 110: 2034–2040.
Lowenberg B, Muus P, Ossenkoppele G, Rousselot P, Cahn JY, Ifrah N et alPhase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood 2011; 118: 6030–6036.
Tsuboi K, Yokozawa T, Sakura T, Watanabe T, Fujisawa S, Yamauchi T et alA phase I study to assess the safety, pharmacokinetics and efficacy of barasertib (AZD1152), an Aurora B kinase inhibitor, in Japanese patients with advanced acute myeloid leukemia. Leuk Res 2011; 35: 1384–1389.
Kantarjian HM, Martinelli G, Jabbour EJ, Quintas-Cardama A, Ando K, Bay JO et alStage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer 2013; 119: 2611–2619.
Kantarjian HM, Sekeres MA, Ribrag V, Rousselot P, Garcia-Manero G, Jabbour EJ et alPhase I study assessing the safety and tolerability of barasertib (AZD1152) with low-dose cytosine arabinoside in elderly patients with AML. Clin Lymphoma Myeloma Leuk 2013; 13: 559–567.
Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J et alBI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res 2009; 15: 3094–3102.
Schoffski P . Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 2009; 14: 559–570.
Strebhardt K . Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 2010; 9: 643–660.
Döhner H, Lübbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM et alRandomized, phase 2 trial comparing low-dose cytarabine with or without volasertib in AML patients not suitable for intensive induction therapy. Blood 2014; 124: 1426–1433.
Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K et alClinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood 2001; 97: 3361–3369.
Lancet JE, Gojo I, Gotlib J, Feldman EJ, Greer J, Liesveld JL et alA phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood 2007; 109: 1387–1394.
Burnett AK, Russell NH, Culligan D, Cavanagh J, Kell J, Wheatley K et alThe addition of the farnesyl transferase inhibitor, tipifarnib, to low dose cytarabine does not improve outcome for older patients with AML. Br J Haematol 2012; 158: 519–522.
Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF et alFirst-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol 2011; 29: 979–985.
Tallman MS, Gilliland DG, Rowe JM . Drug therapy for acute myeloid leukemia. Blood 2005; 106: 1154–1163.
Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N et al In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res 2009; 33: 129–139.
Lim WS, Tardi PG, Dos SN, Xie X, Fan M, Liboiron BD et alLeukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res 2010; 34: 1214–1223.
Lancet JE, Cortes JE, Hogge DE, Tallman M, Kovacsovics T, Damon LE et alPhase 2B randomized study of CPX-351 vs cytarabine (CYT)+daunorubicin (DNR) (7+3 regimen) in newly diagnosed AML patients aged 60–75. Blood 2010; 116: 655.
Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE et alPhase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 2014; 123: 3239–3246.
Ricart AD . Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res 2011; 17: 6417–6427.
Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID . Expression of normal myeloid-associated antigens by acute leukemia cells. Blood 1986; 67: 1048–1053.
Peiper SC, Ashmun RA, Look AT . Molecular cloning, expression, and chromosomal localization of a human gene encoding the CD33 myeloid differentiation antigen. Blood 1988; 72: 314–321.
van der Velden V, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS et alTargeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 2001; 97: 3197–3204.
Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L et alPreliminary results of Southwest Oncology Group Study S0106: an international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood 2009; 114: 790.
Amadori S, Suciu S, Selleslag D, Stasi R, Alimena G, Baila L et alRandomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leukaemia groups (AML-19). Br J Haematol 2010; 149: 376–382.
Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K et alThe addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013; 27: 75–81.
Nand S, Othus M, Godwin JE, Willman CL, Norwood TH, Howard DS et alA phase 2 trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood 2013; 122: 3432–3439.
Gilliland DG, Griffin JD . The roles of FLT3 in hematopoiesis and leukemia. Blood 2002; 100: 1532–1542.
Swords R, Freeman C, Giles F . Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia 2012; 26: 2176–2185.
Ibrahim N, Yu Y, Walsh WR, Yang JL . Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies (review). Oncol Rep 2012; 27: 1303–1311.
Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY et alPhase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 1856–1862.
Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U et alSorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol 2013; 31: 3110–3118.
Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B et alAC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 2009; 114: 2984–2992.
Levis MJ, Perl AE, Dombret H, Dohner H, Steffen B, Rousselot P et alFinal results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood (ASH Annual Meeting Abstracts) 2012; 120: 673.
Perl AE, Dohner H, Rousselot PH, Marie JP, Martinelli G, Shah NP et alEfficacy and safety of quizartinib (AC220) in patients age >=70 years with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia (AML). ASCO Meeting Abstracts 2013; 31: 7023.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et alInhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002; 1: 433–443.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et alPatients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Fischer T, Stone RM, DeAngelo DJ, Galinsky I, Estey E, Lanza C et alPhase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 2010; 28: 4339–4345.
Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J et alCrenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood 2014; 123: 94–100.
Zimmerman EI, Turner DC, Buaboonnam J, Hu S, Orwick S, Roberts MS et alCrenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood 2013; 122: 3607–3615.
Qin T, Castoro R, El AS, Jelinek J, Wang X, Si J et alMechanisms of resistance to decitabine in the myelodysplastic syndrome. PLoS One 2011; 6: e23372.
Jabbour E, Garcia-Manero G, Batty N, Shan J, O'Brien S, Cortes J et alOutcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer 2010; 116: 3830–3834.
Kantarjian HM, Jabbour E, Yee K, Kropf P, O'Connell C, Stock W et alFirst clinical results of a randomized phase 2 study of SGI-110, a novel subcutaneous (SQ) hypomethylating agent (HMA), in adult patients with acute myeloid leukemia (AML). Blood 2013; 122: 497.
Silva G, Cardoso BA, Belo H, Almeida AM . Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms. PLoS One 2013; 8: e53766.
Schaefer EW, Loaiza-Bonilla A, Juckett M, DiPersio JF, Roy V, Slack J et alA phase 2 study of vorinostat in acute myeloid leukemia. Haematologica 2009; 94: 1375–1382.
Walter RB, Medeiros BC, Powell BL, Schiffer CA, Appelbaum FR, Estey EH . Phase II trial of vorinostat and gemtuzumab ozogamicin as induction and post-remission therapy in older adults with previously untreated acute myeloid leukemia. Haematologica 2012; 97: 739–742.
Rosato R, Hock S, Dent P, Dai Y, Grant S . LBH-589 (panobinostat) potentiates fludarabine anti-leukemic activity through a JNK- and XIAP-dependent mechanism. Leuk Res 2012; 36: 491–498.
Xie C, Drenberg C, Edwards H, Caldwell JT, Chen W, Inaba H et alPanobinostat enhances cytarabine and daunorubicin sensitivities in AML cells through suppressing the expression of BRCA1, CHK1, and Rad51. PLoS One 2013; 8: e79106.
Quintas-Cardama A, Kantarjian HM, Ravandi F, Foudray C, Pemmaraju N, Kadia TM et alVery high rates of clinical and cytogenetic response with the combination of the histone deacetylase inhibitor pracinostat (SB939) and 5-azacitidine in high-risk myelodysplastic syndrome. Blood (ASH Annual Meeting Abstracts) 2012; 120: 3821.
Chen K, Huang YH, Chen JL . Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013; 34: 732–740.
Lund K, Adams PD, Copland M . EZH2 in normal and malignant hematopoiesis. Leukemia 2014; 28: 44–49.
Kataoka K, Kurokawa M . Ecotropic viral integration site 1, stem cell self-renewal and leukemogenesis. Cancer Sci 2012; 103: 1371–1377.
Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD et alEfficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366: 2171–2179.
Nicolini FE, Khoury HJ, Akard L, Rea D, Kantarjian H, Baccarani M et alOmacetaxine mepesuccinate for patients with accelerated phase chronic myeloid leukemia with resistance or intolerance to two or more tyrosine kinase inhibitors. Haematologica 2013; 98: e78–e79.
Alvandi F, Kwitkowski VE, Ko CW, Rothmann MD, Ricci S, Saber H et alU.S. Food and drug administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist 2014; 19: 94–99.
Jin J, Wang JX, Chen FF, Wu DP, Hu J, Zhou JF et alHomoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2013; 14: 599–608.
Freeman C, Keane N, Swords R, Giles F . Vosaroxin: a new valuable tool with the potential to replace anthracyclines in the treatment of AML? Expert Opin Pharmacother 2013; 14: 1417–1427.
Lancet JE, Ravandi F, Ricklis RM, Cripe LD, Kantarjian HM, Giles FJ et alA phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia 2011; 25: 1808–1814.
Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N et alON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 2005; 7: 275–286.
Oussenko IA, Holland JF, Reddy EP, Ohnuma T . Effect of ON 01910.Na, an anticancer mitotic inhibitor, on cell-cycle progression correlates with RanGAP1 hyperphosphorylation. Cancer Res 2011; 71: 4968–4976.
Olnes MJ, Shenoy A, Weinstein B, Pfannes L, Loeliger K, Tucker Z et alDirected therapy for patients with myelodysplastic syndromes (MDS) by suppression of cyclin D1 with ON 01910.Na. Leuk Res 2012; 36: 982–989.
Seetharam M, Fan AC, Tran M, Xu L, Renschler JP, Felsher DW et alTreatment of higher risk myelodysplastic syndrome patients unresponsive to hypomethylating agents with ON 01910.Na. Leuk Res 2012; 36: 98–103.
Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson JJ, Wood BL et alComplete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial. Leuk Lymphoma 2009; 50: 1336–1344.
Sekeres MA, Lancet JE, Wood BL, Grove LE, Sandalic L, Sievers EL et alRandomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica 2013; 98: 119–128.
Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS et alSequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 2010; 16: 5303–5311.
Jurcic JG, Ravandi F, Pagel JM, Park JH, Douer D, Estey EH et alPhase I trial of the targeted alpha-particle nano-generator Actinium-225 (225Ac)-lintuzumab (anti-CD33) in combination with low-dose cytarabine (LDAC) for older patients with untreated acute myeloid leukemia (AML). Blood 2013; 122: 1460.
Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H et alSGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013; 122: 1455–1463.
Acknowledgements
We are fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version of this review that reflects the authors’ interpretation and conclusions. Medical writing assistance during the preparation of this review, supported financially by Boehringer Ingelheim Pharmaceuticals, Inc., was provided by Helen Wilkinson of GeoMed, part of KnowledgePoint360, an Ashfield Company. Boehringer Ingelheim was given the opportunity to review for factual accuracy only. This work was supported in part by the MD Anderson Cancer Center Leukemia Support Grant (CCSG) CA016672, philanthropic support from the MD Anderson Cancer Center Moon Shot Program and the Fundacion Ramon Areces.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Montalban-Bravo, G., Garcia-Manero, G. Novel drugs for older patients with acute myeloid leukemia. Leukemia 29, 760–769 (2015). https://doi.org/10.1038/leu.2014.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.244
This article is cited by
-
Exploiting metabolic vulnerabilities for personalized therapy in acute myeloid leukemia
BMC Biology (2019)
-
New drugs in AML: uses and abuses
Leukemia (2018)
-
Akute myeloische Leukämie
Der Onkologe (2017)
-
Quantitative proteomic analysis of histone modifications in decitabine sensitive and resistant leukemia cell lines
Clinical Proteomics (2016)
-
Neue Entwicklungen in der Therapie der akuten myeloischen Leukämie
Im Focus Onkologie (2016)