Abstract
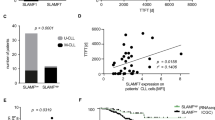
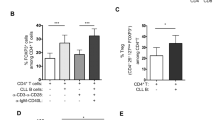
On the basis of somatic hypermutation status of their B-cell antigen receptor (BCR) genes, chronic lymphocytic leukemia (CLL) patients can be divided into unmutated CLL (U-CLL) or mutated CLL (M-CLL). Approximately 30% of CLL patients express a stereotypic BCR, which may indicate that specific antigenic stimulation is driving CLL pathogenesis. Recently, it was reported that BCRs from CLL cells are capable of antigen-independent, cell-autonomous signaling, through recognition of an internal framework 2 (FR2) BCR epitope. We hypothesized that the level of cell-autonomous signaling may differ between CLL subgroups. Therefore, we analyzed Ca2+ signaling in a series of primary stereotypic or heterogeneous U-CLL and M-CLL (n=68) and healthy controls (n=14). We confirmed that basal Ca2+ signaling in CLL cells is higher than in normal B cells. Interestingly, we found that basal signaling was particularly increased in M-CLL. The degree of basal signaling did not correlate with membrane immunoglobulin levels, HCDR3 characteristics or FR2/FR3 sequence. We conclude that the level of basal Ca2+ signaling is not uniformly enhanced in CLL B cells, but is associated with CLL immunoglobulin heavy chain V mutational status, reflecting a distinct cellular origin and possibly a different anergic state induced by repetitive or continuous antigen binding in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–249.
Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999; 94: 1840–1847.
Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK . Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999; 94: 1848–1854.
Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood 2012; 119: 4467–4475.
Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest 2005; 115: 1636–1643.
Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med 2008; 14: 665–674.
Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood 2010; 115: 3907–3915.
Myhrinder AL, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood 2008; 111: 3838–3848.
Zwick C, Fadle N, Regitz E, Kemele M, Stilgenbauer S, Buhler A et al. Autoantigenic targets of B-cell receptors derived from chronic lymphocytic leukemias bind to and induce proliferation of leukemic cells. Blood 2013; 121: 4708–4717.
Ghia EM, Widhopf GF 2nd, Rassenti LZ, Kipps TJ . Analyses of recombinant stereotypic IGHV3-21-encoded antibodies expressed in chronic lymphocytic leukemia. J Immunol 2011; 186: 6338–6344.
Hoogeboom R, Wormhoudt TA, Schipperus MR, Langerak AW, Dunn-Walters DK, Guikema JE et al. A novel chronic lymphocytic leukemia subset expressing mutated IGHV3-7-encoded rheumatoid factor B-cell receptors that are functionally proficient. Leukemia 2013; 27: 738–740.
Kostareli E, Gounari M, Janus A, Murray F, Brochet X, Giudicelli V et al. Antigen receptor stereotypy across B-cell lymphoproliferations: the case of IGHV4-59/IGKV3-20 receptors with rheumatoid factor activity. Leukemia 2012; 26: 1127–1131.
Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med 2013; 210: 59–70.
Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012; 489: 309–312.
Binder M, Muller F, Frick M, Wehr C, Simon F, Leistler B et al. CLL B-cell receptors can recognize themselves: alternative epitopes and structural clues for autostimulatory mechanisms in CLL. Blood 2013; 121: 239–241.
van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17: 2257–2317.
Langerak AW, Davi F, Ghia P, Hadzidimitriou A, Murray F, Potter KN et al. Immunoglobulin sequence analysis and prognostication in CLL: guidelines from the ERIC review board for reliable interpretation of problematic cases. Leukemia 2011; 25: 979–984.
Thompson JD, Higgins DG, Gibson TJ . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22: 4673–4680.
Chen X, Hale GA, Neale GA, Knowles J, Barfield RC, Wang YD et al. A novel approach for the analysis of T-cell reconstitution by using a T-cell receptor beta-based oligonucleotide microarray in hematopoietic stem cell transplantation. Exp Hematol 2007; 35: 831–841.
Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK . Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood 2007; 109: 4424–4431.
Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T . Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia 2007; 21: 2442–2451.
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC . Predominant autoantibody production by early human B cell precursors. Science 2003; 301: 1374–1377.
Sutton LA, Kostareli E, Hadzidimitriou A, Darzentas N, Tsaftaris A, Anagnostopoulos A et al. Extensive intraclonal diversification in a subgroup of chronic lymphocytic leukemia patients with stereotyped IGHV4-34 receptors: implications for ongoing interactions with antigen. Blood 2009; 114: 4460–4468.
Gauld SB, Benschop RJ, Merrell KT, Cambier JC . Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol 2005; 6: 1160–1167.
Zikherman J, Parameswaran R, Weiss A . Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 2012; 489: 160–164.
Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood 2008; 112: 188–195.
Apollonio B, Scielzo C, Bertilaccio MT, Ten Hacken E, Scarfo L, Ranghetti P et al. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood 2013; 121: 3879–3888,, S3871-S3878.
Contri A, Brunati AM, Trentin L, Cabrelle A, Miorin M, Cesaro L et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest 2005; 115: 369–378.
Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res 2009; 69: 5424–5432.
Cesano A, Perbellini O, Evensen E, Chu CC, Cioffi F, Ptacek J et al. Association between B-cell receptor responsiveness and disease progression in B-cell chronic lymphocytic leukemia: results from single cell network profiling studies. Haematologica 2013; 98: 626–634.
Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117: 6287–6296.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42.
Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med 2004; 351: 893–901.
Coscia M, Pantaleoni F, Riganti C, Vitale C, Rigoni M, Peola S et al. IGHV unmutated CLL B cells are more prone to spontaneous apoptosis and subject to environmental prosurvival signals than mutated CLL B cells. Leukemia 2011; 25: 828–837.
Acknowledgements
We thank Marjolein de Bruijn for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Muggen, A., Pillai, S., Kil, L. et al. Basal Ca2+ signaling is particularly increased in mutated chronic lymphocytic leukemia. Leukemia 29, 321–328 (2015). https://doi.org/10.1038/leu.2014.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.188
This article is cited by
-
ACOX1-mediated peroxisomal fatty acid oxidation contributes to metabolic reprogramming and survival in chronic lymphocytic leukemia
Leukemia (2024)
-
Targeting metabolic reprogramming in chronic lymphocytic leukemia
Experimental Hematology & Oncology (2022)
-
Proteogenomics refines the molecular classification of chronic lymphocytic leukemia
Nature Communications (2022)
-
STIM1 at the plasma membrane as a new target in progressive chronic lymphocytic leukemia
Journal for ImmunoTherapy of Cancer (2019)
-
CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes
Cellular & Molecular Immunology (2018)