Abstract
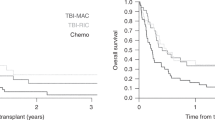
Children with acute lymphoblastic leukemia (ALL) and high minimal residual disease (MRD) levels after initial chemotherapy have a poor clinical outcome. In this prospective, single arm, Phase 2 trial, 111 Dutch and Australian children aged 1–18 years with newly diagnosed, t(9;22)-negative ALL, were identified among 1041 consecutively enrolled patients as high risk (HR) based on clinical features or high MRD. The HR cohort received the AIEOP-BFM (Associazione Italiana di Ematologia ed Oncologia Pediatrica (Italy)–Berlin-Frankfurt-Münster ALL Study Group) 2000 ALL Protocol I, then three novel HR chemotherapy blocks, followed by allogeneic transplant or chemotherapy. Of the 111 HR patients, 91 began HR treatment blocks, while 79 completed the protocol. There were 3 remission failures, 12 relapses, 7 toxic deaths in remission and 10 patients who changed protocol due to toxicity or clinician/parent preference. For the 111 HR patients, 5-year event-free survival (EFS) was 66.8% (±5.5) and overall survival (OS) was 75.6% (±4.3). The 30 patients treated as HR solely on the basis of high MRD levels had a 5-year EFS of 63% (±9.4%). All patients experienced grade 3 or 4 toxicities during HR block therapy. Although cure rates were improved compared with previous studies, high treatment toxicity suggested that novel agents are needed to achieve further improvement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schrappe M, Nachman J, Hunger S, Schmiegelow K, Conter V, Masera G et al. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985-2000). Leukemia 2010; 24 (2): 253–254.
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360: 2730–2741.
Van Dongen JJM, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 1998; 352: 1731–1738.
Marshall GM, Haber M, Kwan E, Zhu L, Ferrara D, Xue C et al. Importance of minimal residual disease testing during the second year of therapy for children with acute lymphoblastic leukaemia. J Clin Oncol 2003; 21 (4): 704–709.
Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008; 111 (9): 4477–4489.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010; 115: 3206–3214.
Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grümayer R, Möricke A et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AEIOP-BFM-ALL 2000 Study. Blood 2011; 118 (8): 2077–2084.
Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of Trial ALL-REZ BFM 90. J Clin Oncol 2010; 28 (14): 2339–2347.
Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet 2010; 376: 2009–2017.
Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood 2002; 100: 52–58.
Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D et al. Risk of relapse of childhood acute lymphoblastic leukaemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol 2009; 27 (31): 5168–5174.
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood 2008; 111 (12): 5477–5485.
Choi S, Henderson MJ, Kwan E, Beesley AH, Sutton R, Bahar AY et al. Relapse in children with acute lymphoblastic leukaemia involving selection of a pre-existing drug resistant sub-clone. Blood 2007; 110 (2): 632–639.
Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M et al. Improved early EFS with imatinib in philadelphia chromosome-positive acute lymphoblastic leukemia: a Children’s Oncology Group study. J Clin Oncol 2009; 27: 5175–5181.
Ribera J-M, Ortega J-J, Oriol A, Bastida P, Calvo C, Pérez-Hurtado JM et al. Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol 2007; 25 (1): 16–24.
Schrauder A, Reiter A, Gadner H, Niethammer D, Klingebiel T, Kremens B et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol 2006; 24 (36): 5742–5749.
Aricó M, Schrappe M, Hunger SP, Carroll WL, Conter V, Galimberti S et al. Clinical outcome of children with newly diagnosed philadelphia chromosome–positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol 2010; 28 (31): 4755–4761.
Brown RA, Herzig RH, Wolff SN, Frei-Lahr D, Pineiro L, Bolwell BJ et al. High-dose etoposide and cyclophosphamide without bone marrow transplantation for resistant hematologic malignancy. Blood 1990; 76 (3): 473–479.
Morland BJ, Shaw PJ. . Induction toxicity of a modified Memorial Sloan-Kettering New York II protocol in children with relapsed adult lymphoblastic leukemia: a single institution study. Med Ped Oncol 1996; 27: 139–144.
Feldman EJ, Albert DS, Arlin Z, Ahmed T, Mittelman A, Baskind P et al. Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia. J Clin Oncol 1993; 11 (10): 2002–2009.
Arlin ZA, Feldman EJ, Finger LR, Ahmed T, Mittelman A, Cook P et al. Short course high-dose mitoxantrone with high-dose cytarabine is effective therapy for adult lymphoblastic leukemia. Leukemia 1991; 5 (8): 712–714.
Visani G, Tosi P, Zinzani PL, Manfroi S, Ottaviani E, Cenacchi A et al. FLAG (fludarabine, cytarabine, G-CSF) as second line therapy for acute lymphoblastic leukemia with myeloid antigen expression: in vitro and in vivo effects. Eur J Haem 1996; 56 (5): 308–312.
McCarthy AJ, Pitcher LA, Hann IM, Oakhill A. . FLAG (fludarabine, high-dose cytarabine and G-CSF) for refractory and relapsed high risk acute leukemia in children. Med Ped Oncol 1999; 34 (2): 411–415.
Flohr T, Schrauder A, Cazzaniga G, Panzer-Grümayer R, van der Velden V, Fischer S et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia 2008; 22: 771–782.
Sutton R, Bahar AY, Kwan E, Giles JE, Venn NC, Tran S et al. Improving minimal residual disease detection in precursor-B-All based on immunoglobulin kappa and heavy chain gene rearrangements. Leukemia 2008; 22: 2265–2267.
Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Brit J Haem 2009; 146: 292–299.
van der Velden VHJ, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007; 21: 604–611.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13 (3): 176–181.
Nachman J, Sather HN, Cherlow JM, Sensel MG, Gaynon PS, Lukens JN et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: a report from the Children’s Cancer Group. J Clin Oncol 1998; 16: 920–930.
Aricò M, Valsecchi MG, Conter V, Rizzari C, Pession A, Messina C et al. Improved outcome in high-risk childhood acute lymphoblastic leukemia defined by prednisone-poor response treated with double Berlin-Frankfurt-Muenster protocol II. Blood 2002; 100: 420–426.
Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 2007; 109: 896–904.
Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood 2008; 111: 2548–2555.
Bordigoni P, Vernant JP, Souillet G, Gluckman E, Marininchi D, Milpied N et al. Allogeneic bone marrow transplantation for children with acute lymphoblastic leukemia in first remission: a cooperative study of the Groupe d'Etude de la Greffe de Moelle Osseuse. J Clin Oncol 1989; 7: 747–753.
Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C et al. Chemotherapy versus allogeneic transplantation for very high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 2005; 366: 635–642.
Satwani P, Sather H, Ozkaynak F, Heerema NA, Schultz KR, Sanders J et al. Allogeneic bone marrow transplantation in first remission for children with ultra-high-risk features of acute lymphoblastic leukemia: a Children’s Oncology Group study report. Biol Blood Marrow Transplant 2007; 13: 218–227.
Acknowledgements
We thank Prof Martin Schrappe and the I-BFM for their support. This work was supported by the National Health and Medical Research Council of Australia, Cancer Council New South Wales, Steven Walter Children’s Cancer Foundation, Cancer Institute New South Wales and Leukemia Foundation.
Author contributions
Conception and design: LDP, GMM, MDN and RP. Administrative support: RS, AN, HG, SC, HM and VH. Provision of study materials or patients: LDP, RS, NCV, VHV, HB, ESJMB, RME, PMH, GJLK, ES, JD, TL, MDN, MH, TR, A, RS, RP and GMM. Collection and assembly of data: GMM, LDP, RS, AN, HG-K , NCV, VHV, HM, VH, MDN, MH, TR, FA and RP. Data analysis and interpretation: GMM, LDP, RS, AN, HG-K, NCV, VHV, SC, HM, VH, MDN, MH, TR, FA, RS and RP. Manuscript writing: GMM, LDP, RS, AN and RP. Final approval of manuscript: All the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Marshall, G., Dalla Pozza, L., Sutton, R. et al. High-risk childhood acute lymphoblastic leukemia in first remission treated with novel intensive chemotherapy and allogeneic transplantation. Leukemia 27, 1497–1503 (2013). https://doi.org/10.1038/leu.2013.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.44
Keywords
This article is cited by
-
Outcomes for paediatric acute leukaemia patients admitted to the paediatric intensive care unit
European Journal of Pediatrics (2022)
-
Prospective longitudinal evaluation of treatment-related toxicity and health-related quality of life during the first year of treatment for pediatric acute lymphoblastic leukemia
BMC Cancer (2022)
-
Whole-genome sequencing facilitates patient-specific quantitative PCR-based minimal residual disease monitoring in acute lymphoblastic leukaemia, neuroblastoma and Ewing sarcoma
British Journal of Cancer (2022)
-
Alternative donor peripheral blood stem cell transplantation for the treatment of high-risk refractory and/or relapsed childhood acute leukemia: a randomized trial
Experimental Hematology & Oncology (2020)
-
Examining treatment responses of diagnostic marrow in murine xenografts to predict relapse in children with acute lymphoblastic leukaemia
British Journal of Cancer (2020)