Abstract
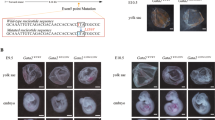
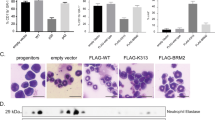
Transient leukemia (TL) is evident in 5–10% of all neonates with Down syndrome (DS) and associated with N-terminal truncating GATA1 mutations (GATA1s). Here we report that TL-cell clones generate abundant eosinophils in a substantial fraction of patients. Sorted eosinophils from patients with TL and eosinophilia carried the same GATA1s mutations as sorted TL blasts, consistent with their clonal origin. TL blasts exhibited a genetic program characteristic of eosinophils and differentiated along the eosinophil lineage in vitro. Similarly, ectopic expression of Gata1s, but not Gata1, in wild-type CD34+-hematopoietic stem and progenitor cells induced hyperproliferation of eosinophil promyelocytes in vitro. Although GATA1s retained the function of GATA1 to induce eosinophil genes by occupying their promoter regions, GATA1s was impaired in its ability to repress oncogenic MYC and the pro-proliferative E2F transcription network. Chromatin Immunoprecipitation Sequencing (ChIP-seq) indicated reduced GATA1s occupancy at the MYC promoter. Knockdown of MYC, or the obligate E2F-cooperation partner DP1, rescued the GATA1s-induced hyperproliferative phenotype. In agreement, terminal eosinophil maturation was blocked in Gata1Δe2 knockin mice, exclusively expressing Gata1s, leading to accumulation of eosinophil precursors in blood and bone marrow. These data suggest a direct relationship between the N-terminal truncating mutations of GATA1 and clonal eosinophilia in DS patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hasle H, Clemmensen IH, Mikkelsen M . Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet 2000; 355: 165–169.
Hasle H, Abrahamsson J, Arola M, Karow A, O'Marcaigh A, Reinhardt D et al. Myeloid leukemia in children 4 years or older with Down syndrome often lacks GATA1 mutation and cytogenetics and risk of relapse are more akin to sporadic AML. Leukemia 2008; 22: 1428–1430.
Klusmann JH, Creutzig U, Zimmermann M, Dworzak M, Jorch N, Langebrake C et al. Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood 2008; 111: 2991–2998.
Lightfoot J, Hitzler JK, Zipursky A, Albert M, Macgregor PF . Distinct gene signatures of transient and acute megakaryoblastic leukemia in Down syndrome. Leukemia 2004; 18: 1617–1623.
Blink M, Buitenkamp TD, van den Heuvel-Eibrink MM, Danen-van Oorschot AA, de Haas V, Reinhardt D et al. Frequency and prognostic implications of JAK 1-3 aberrations in Down syndrome acute lymphoblastic and myeloid leukemia. Leukemia 2011; 25: 1365–1368.
Klusmann JH, Reinhardt D, Hasle H, Kaspers GJ, Creutzig U, Hahlen K et al. Janus kinase mutations in the development of acute megakaryoblastic leukemia in children with and without Down's syndrome. Leukemia 2007; 21: 1584–1587.
Kiyoi H, Yamaji S, Kojima S, Naoe T . JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. Leukemia 2007; 21: 574–576.
Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet 2002; 32: 148–152.
Dore LC, Crispino JD . Transcription factor networks in erythroid cell and megakaryocyte development. Blood 2011; 118: 231–239.
Rylski M, Welch JJ, Chen YY, Letting DL, Diehl JA, Chodosh LA et al. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol 2003; 23: 5031–5042.
Weiss MJ, Orkin SH . GATA transcription factors: key regulators of hematopoiesis. Exp Hematol 1995; 23: 99–107.
Burda P, Laslo P, Stopka T . The role of PU.1 and GATA-1 transcription factors during normal and leukemogenic hematopoiesis. Leukemia 2010; 24: 1249–1257.
Klusmann JH, Godinho FJ, Heitmann K, Maroz A, Koch ML, Reinhardt D et al. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev 2010; 24: 1659–1672.
Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH . Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet 2005; 37: 613–619.
Ge Y, LaFiura KM, Dombkowski AA, Chen Q, Payton SG, Buck SA et al. The role of the proto-oncogene ETS2 in acute megakaryocytic leukemia biology and therapy. Leukemia 2008; 22: 521–529.
Stankiewicz MJ, Crispino JD . AKT collaborates with ERG and Gata1s to dysregulate megakaryopoiesis and promote AMKL. Leukemia 2013; 27: 1339–1347.
Harigae H, Takahashi S, Suwabe N, Ohtsu H, Gu L, Yang Z et al. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells 1998; 3: 39–50.
Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, Kato Y et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med 2002; 195: 1379–1386.
Takahashi S, Komeno T, Suwabe N, Yoh K, Nakajima O, Nishimura S et al. Role of GATA-1 in proliferation and differentiation of definitive erythroid and megakaryocytic cells in vivo. Blood 1998; 92: 434–442.
Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 2002; 195: 1387–1395.
Nei Y, Obata-Ninomiya K, Tsutsui H, Ishiwata K, Miyasaka M, Matsumoto K et al. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci USA 2013; 110: 18620–18625.
Du J, Stankiewicz MJ, Liu Y, Xi Q, Schmitz JE, Lekstrom-Himes JA et al. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem 2002; 277: 43481–43494.
Uhm TG, Kim BS, Chung IY . Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res 2012; 4: 68–79.
Heyworth C, Pearson S, May G, Enver T . Transcription factor-mediated lineage switching reveals plasticity in primary committed progenitor cells. EMBO J 2002; 21: 3770–3781.
Eguchi M, Sakaibara H, Suda J, Ozawa T, Hayashi Y, Sato T et al. Ultrastructural and ultracytochemical differences between transient myeloproliferative disorder and megakaryoblastic leukaemia in Down's syndrome. Br J Haematol 1989; 73: 315–322.
Miyauchi J, Ito Y, Tsukamoto K, Takahashi H, Ishikura K, Sugita K et al. Blasts in transient leukaemia in neonates with Down syndrome differentiate into basophil/mast-cell and megakaryocyte lineages in vitro in association with down-regulation of truncated form of GATA1. Br J Haematol 2010; 148: 898–909.
Suda T, Suda J, Miura Y, Hayashi Y, Eguchi M, Tadokoro K et al. Clonal analysis of basophil differentiation in bone marrow cultures from a Down's syndrome patient with megakaryoblastic leukemia. Blood 1985; 66: 1278–1283.
Stankov MV, El Khatib M, Kumar Thakur B, Heitmann K, Panayotova-Dimitrova D, Schoening J et al. Histone deacetylase inhibitors induce apoptosis in myeloid leukemia by suppressing autophagy. Leukemia 2014; 28: 577–588.
Emmrich S, Henke K, Hegermann J, Ochs M, Reinhardt D, Klusmann JH . miRNAs can increase the efficiency of ex vivo platelet generation. Ann Hematol 2012; 91: 1673–1684.
Weber K, Bartsch U, Stocking C, Fehse B . A multicolor panel of novel lentiviral ‘gene ontology’ (LeGO) vectors for functional gene analysis. Mol Ther 2008; 16: 698–706.
Klusmann JH, Li Z, Bohmer K, Maroz A, Koch ML, Emmrich S et al. miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev 2010; 24: 478–490.
Hennig C, Adams N, Hansen G . A versatile platform for comprehensive chip-based explorative cytometry. Cytometry A 2009; 75: 362–370.
Kim J, Cantor AB, Orkin SH, Wang J . Use of in vivo biotinylation to study protein-protein and protein-DNA interactions in mouse embryonic stem cells. Nat Protoc 2009; 4: 506–517.
Xu J, Shao Z, Glass K, Bauer DE, Pinello L, Van Handel B et al. Combinatorial assembly of developmental stage-specific enhancers controls gene expression programs during human erythropoiesis. Dev Cell 2012; 23: 796–811.
Rodriguez P, Braun H, Kolodziej KE, de Boer E, Campbell J, Bonte E et al. Isolation of transcription factor complexes by in vivo biotinylation tagging and direct binding to streptavidin beads. Methods Mol Biol 2006; 338: 305–323.
Hamaguchi-Tsuru E, Nobumoto A, Hirose N, Kataoka S, Fujikawa-Adachi K, Furuya M et al. Development and functional analysis of eosinophils from murine embryonic stem cells. Br J Haematol 2004; 124: 819–827.
Bourquin JP, Subramanian A, Langebrake C, Reinhardt D, Bernard O, Ballerini P et al. Identification of distinct molecular phenotypes in acute megakaryoblastic leukemia by gene expression profiling. Proc Natl Acad Sci USA 2006; 103: 3339–3344.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Nakajima T, Matsumoto K, Suto H, Tanaka K, Ebisawa M, Tomita H et al. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood 2001; 98: 1127–1134.
Toki T, Kanezaki R, Adachi S, Fujino H, Xu G, Sato T et al. The key role of stem cell factor/KIT signaling in the proliferation of blast cells from Down syndrome-related leukemia. Leukemia 2009; 23: 95–103.
Bohn G, Allroth A, Brandes G, Thiel J, Glocker E, Schaffer AA et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med 2007; 13: 38–45.
Kulessa H, Frampton J, Graf T . GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev 1995; 9: 1250–1262.
Tanaka H, Matsumura I, Nakajima K, Daino H, Sonoyama J, Yoshida H et al. GATA-1 blocks IL-6-induced macrophage differentiation and apoptosis through the sustained expression of cyclin D1 and bcl-2 in a murine myeloid cell line M1. Blood 2000; 95: 1264–1273.
Yamaguchi Y, Zon LI, Ackerman SJ, Yamamoto M, Suda T . Forced GATA-1 expression in the murine myeloid cell line M1: induction of c-Mpl expression and megakaryocytic/erythroid differentiation. Blood 1998; 91: 450–457.
Voehringer D, Shinkai K, Locksley RM . Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 2004; 20: 267–277.
Dyer KD, Garcia-Crespo KE, Percopo CM, Sturm EM, Rosenberg HF . Protocols for identifying, enumerating, and assessing mouse eosinophils. Methods Mol Biol 2013; 1032: 59–77.
Voehringer D, Van Rooijen N, Locksley RM . Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol 2007; 81: 1434–1444.
Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 2002; 16: 245–256.
Kadri Z, Shimizu R, Ohneda O, Maouche-Chretien L, Gisselbrecht S, Yamamoto M et al. Direct binding of pRb/E2F-2 to GATA-1 regulates maturation and terminal cell division during erythropoiesis. PLoS Biol 2009; 7: e1000123.
Fujiwara T, O'Geen H, Keles S, Blahnik K, Linnemann AK, Kang YA et al. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol Cell 2009; 36: 667–681.
Yu M, Riva L, Xie H, Schindler Y, Moran TB, Cheng Y et al. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell 2009; 36: 682–695.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917.
Thalmeier K, Synovzik H, Mertz R, Winnacker EL, Lipp M . Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev 1989; 3: 527–536.
Sears R, Ohtani K, Nevins JR . Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol 1997; 17: 5227–5235.
Mateyak MK, Obaya AJ, Sedivy JM . c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol 1999; 19: 4672–4683.
Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet 2006; 38: 807–812.
Worth LL, Zipursky A, Christensen H, Tubergen D . Transient leukemia with extreme basophilia in a phenotypically normal infant with blast cells containing a pseudodiploid clone, 46,XY i(21)(q10). J Pediatr Hematol Oncol 1999; 21: 63–66.
Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF . Differentiation in vitro of hybrid eosinophil/basophil granulocytes: autocrine function of an eosinophil developmental intermediate. J Exp Med 1995; 182: 49–57.
Acknowledgements
The authors thank J Strouboulis and E Karkoulia for providing BioGata1/Gata1s ESCs. J Schoening for general lab support; Drs K Weber and B Fehse for providing plasmids; Dr A Mirenska for chip cytometry data analysis; Dr R Geffers for microarray analysis. AM, LS, SE and TD were supported by the Hannover Biomedical Research School. JHK is a fellow of the Emmy Noether-Programme from the German Research Foundation (KL-2374/2-1). This work was supported by a grant to JHK from the German Research Foundation (KL-2374/1-1).
Author contributions
AM, KR and TD performed experiments; LS, SE, JX, ZS and GJ performed experiments and analyzed results. AM and JHK analyzed and interpreted results, prepared the figures and wrote the manuscript; JHK designed the research. DR and JH provided patient material and revised the manuscript. IR, CH, KR, PV, ZL and SO analyzed and interpreted results and revised the manuscript. GH provided access and support to laboratory equipment and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CH has filed patents regarding chip cytometry. The other authors declare no competing financial interests.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Maroz, A., Stachorski, L., Emmrich, S. et al. GATA1s induces hyperproliferation of eosinophil precursors in Down syndrome transient leukemia. Leukemia 28, 1259–1270 (2014). https://doi.org/10.1038/leu.2013.373
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.373