Abstract
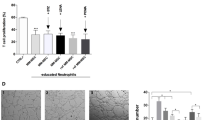
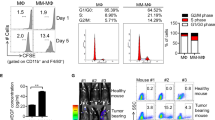
The role of cancer-associated fibroblasts (CAFs) has not been previously studied in multiple myeloma (MM). Here, cytofluorimetric analysis revealed higher proportions of bone marrow (BM) CAFs in patients with active MM (both at diagnosis and relapse) compared with patients in remission or those with monoclonal gammopathy of undetermined significance or deficiency anemia (controls). CAFs from MM patients produced increased levels of transforming growth factor-β, interleukin-6, stromal cell-derived factor-1α, insulin-like growth factor-1, vascular endothelial growth factor and fibroblast growth factor-2 and displayed an activated and heterogeneous phenotype, which supported their origin from resident fibroblasts, endothelial cells and hematopoietic stem and progenitor cells via the endothelial–mesenchymal transition as well as mesenchymal stem cells via the mesenchymal transition, as both of these processes are induced by MM cells and CAFs. Active MM CAFs fostered chemotaxis, adhesion, proliferation and apoptosis resistance in MM cells through cytokine signals and cell-to-cell contact, which were inhibited by blocking CXCR4, several integrins and fibronectin. MM cells also induced the CAFs proliferation. In syngeneic 5T33MM and xenograft mouse models, MM cells induced the expansion of CAFs, which, in turn, promoted MM initiation and progression as well as angiogenesis. In BM biopsies from patients and mice, nests of CAFs were found in close contact with MM cells, suggesting a supportive niche. Therefore, the targeting of CAFs in MM patients may be envisaged as a novel therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Tlsty TD, Hein PW . Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev 2001; 11: 54–59.
Li H, Fan X, Houghton J . Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007; 101: 805–815.
Orimo A, Weinberg RA . Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 2006; 5: 1597–1601.
Kalluri R, Zeisberg M . Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401.
Franco OE, Shaw AK, Strand DW, Hayward SW . Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 2010; 21: 33–39.
Shimoda M, Mellody KT, Orimo A . Carcinoma-associated fibroblasts are a rate-limiting determinant for tumor progression. Semin Cell Dev Biol 2010; 21: 19–25.
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 Secretion. Cell 2005; 121: 335–348.
Erez N, Truitt M, Olson P, Arron ST, Hanahan D . Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010; 17: 135–147.
De Wever O, Demetter P, Mareel M, Bracke M . Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123: 2229–2238.
Orimo A, Weinberg RA . Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Ther 2007; 6: 618–619.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R . Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 2006; 5: 1640–1646.
Anderberg C, Pietras K . On the origin of cancer-associated fibroblasts. Cell Cycle 2009; 8: 1461–1462.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G . The myofibroblasts: one function, multiple origins. Am J Pathol 2007; 170: 1807–1816.
Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP et al. Carcinoma-associated fibroblasts-like differentiation of human mesenchymal stem cells. Cancer Res 2008; 68: 4331–4339.
Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 2004; 64: 8492–8495.
Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R . Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 2007; 67: 10123–10128.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428.
Hinz B, Gabbiani G . Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 2003; 14: 538–546.
Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature 2004; 429: 83–86.
Kumar S, Weaver VM . Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 2009; 28: 113–127.
Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 2010; 107: 20009–20014.
Ria R, Reale A, De Luisi A, Ferrucci A, Moschetta M, Vacca A . Bone marrow angiogenesis and progression in multiple myeloma. Am J Blood Res 2011; 1: 76–89.
Vacca A, Ribatti D . Bone marrow angiogenesis in multiple myeloma. Leukemia 2006; 20: 193–199.
Basile A, Moschetta M, Ditonno P, Ria R, Marech I, De Luisi A et al. Pentraxin 3 (PTX3) inhibits plasma cell/stromal cell cross-talk in the bone marrow of multiple myeloma patients. J Pathol 2013; 229: 87–98.
Berardi S, Caivano A, Ria R, Nico B, Savino R, Terracciano R et al. Four proteins governing overangiogenic endothelial cell phenotype in patients with multiple myeloma are plausible therapeutic targets. Oncogene 2012; 31: 2258–2269.
Santos AM, Jung J, Aziz N, Kissil JL, Purè E . Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 2009; 119: 3613–3625.
Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 2002; 46: 3349–3360.
Ghobrial IM . Myeloma as a model for the process of metastasis: implications for therapy. Blood 2012; 120: 20–30.
Ria R, Piccoli C, Cirulli T, Falzetti F, Mangialardi G, Guidolin D et al. Endothelial differentiation of hematopoietic stem and progenitor cells from patients with multiple myeloma. Clin Cancer Res 2008; 14: 1678–1685.
Xu S, Menu E, De Becker A, Van Camp B, Vanderkerken K, Van Riet I . Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells 2012; 30: 266–279.
Möller C, Strömberg T, Juremalm M, Nilsson K, Nilsson G . Expression and function of chemokine receptors in human multiple myeloma. Leukemia 2003; 17: 203–210.
Damiano JS, Dalton WS . Integrin-mediated drug resistance in multiple myeloma. Leuk Lymphoma 2000; 38: 71–81.
Saito RA, Micke P, Paulsson J, Augsten M, Peña C, Jönsson P et al. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res 2010; 70: 2644–2654.
Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest 2012; 122: 3603–3617.
Östman A, Augsten M . Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev 2009; 19: 67–73.
Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood 1996; 87: 1928–1938.
Hayashi T, Hideshima T, Nguyen AN, Munoz O, Podar K, Hamasaki M et al. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res 2004; 10: 7540–7546.
Markwald RR, Fitzharris TP, Smith WN . Sturctural analysis of endocardial cytodifferentiation. Dev Biol 1975; 42: 160–180.
van Meeteren LA, ten Dijke P . Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res 2012; 347: 177–186.
Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC . Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res 2010; 86: 506–515.
Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009; 4: e4992.
Wang LH, Yang XY, Zhang X, Farrar WL . Inhibition of adhesive interaction between multiple myeloma and bone marrow stromal cells by PPARgamma cross talk with NF-kappaB and C/EBP. Blood 2007; 110: 4373–4384.
Teoh G, Anderson KC . Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol Oncol Clin North Am 1997; 11: 27–42.
Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A . The history of the angiogenic switch concept. Leukemia 2007; 21: 44–52.
Damiano JS, Hazlehurst LA, Dalton WS . Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic mielogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia 2001; 15: 1232–1239.
Damiano JS . Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets 2002; 2: 37–43.
Nefedova Y, Landowski TH, Dalton WS . Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003; 17: 1175–1182.
Vacca A, Ria R, Presta M, Ribatti D, Iurlaro M, Merchionne F et al. alpha(v)beta(3) integrin engagement modulates cell adhesion, proliferation, and protease secretion in human lymphoid tumor cells. Exp Hematol 2001; 29: 993–1003.
van Riet I, de Greef C, del Favero H, Demanet C, Van Camp B . Production of fibronectin and adherence to fibronectin by human myeloma cell lines. Br J Haematol 1994; 87: 258–265.
Tancred TM, Belch AR, Reiman T, Pilarski LM, Kirshner J . Altered expression of fibronectin and collagens I and IV in multiple myeloma and monoclonal gammopathy of undetermined significance. J Histochem Cytochem 2009; 57: 239–247.
Acknowledgements
We are grateful to K De Veirman (Vrije Universiteit Brussels) for help with the cytofluorimetric experiments in syngeneic 5T33 mice. This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC); an Investigator Grant (number 10099 to AV); the Special Program Molecular Clinical Oncology 5 per 1000 (number 9965 to AV), Milan; the European Commission’s Seventh Framework Programme (EU FPT7) OVER-MyR (number 278706 to AV and KV) and OPTATIO (number 278570 to DR); and the Ministry of Health (Progetto PRIN 2009 to RR and 2012 to AV), Rome, Italy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Frassanito, M., Rao, L., Moschetta, M. et al. Bone marrow fibroblasts parallel multiple myeloma progression in patients and mice: in vitro and in vivo studies. Leukemia 28, 904–916 (2014). https://doi.org/10.1038/leu.2013.254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.254
Keywords
This article is cited by
-
The emerging studies on mesenchymal progenitors in the long bone
Cell & Bioscience (2023)
-
Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma
Scientific Reports (2023)
-
CXCL12/CXCR4 axis supports mitochondrial trafficking in tumor myeloma microenvironment
Oncogenesis (2022)
-
Presence of bone marrow fibrosis in multiple myeloma may predict extramedullary disease
International Journal of Hematology (2022)
-
Establishment and characterization of HXWMF-1: the first mouse fibroblastic tumor cell line derived from leukemia-associated fibroblasts
Cancer Cell International (2021)