Abstract
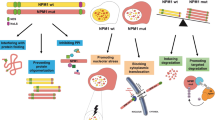
Mutations in exon 12 of the nucleophosmin (NPM1) gene (NPMc+ (NPM1 COOH terminal mutations)) define a distinct subset of acute myelogenous leukemias (AMLs), in which the NPMc+ protein localizes aberrantly to the leukemic cell cytoplasm. We have found that introduction of the most common NPMc+ variant into K562 and 32D cells sensitizes these cells to apoptosis induced by drugs such as bortezomib and arsenic trioxide (ATO) that induce reactive oxygen species (ROS) formation, and that cytotoxicity is prevented in the presence of N-acetyl-L-cysteine (NAC), an ROS scavenger. The substitution of tryptophan 288 (W288) by cysteine occurs in the great majority of NPM1c+ mutations. Mutagenesis of cysteine 288 to alanine re-localizes NPMc+ from the cytoplasm to the nucleolus and attenuates the sensitivity of cells expressing this mutation to bortezomib and ATO. Primary AML cells expressing NPMc+ are also significantly more sensitive than other AML cells to apoptosis induced by both drugs at pharmacologically achievable doses. We conclude that the presence of a cysteine moiety at position 288 results in the cytoplasmic localization of NPM1c+ and the increased sensitivity to bortezomib and ATO. These data suggest that bortezomib and ATO may have increased therapeutic efficacy in NPM1c+ leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Colombo E, Alcalay M, Pelicci PG . Nucleophosmin and its complex network: a possible therapeutic target in hematological diseases. Oncogene 2011; 30: 2595–2609.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood 2011; 117: 1109–1120.
Falini B, Gionfriddo I, Cecchetti F, Ballanti S, Pettirossi V, Martelli MP . Acute myeloid leukemia with mutated nucleophosmin (NPM1): any hope for a targeted therapy? Blood Rev 2011; 25: 247–254.
Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 2006; 107: 4514–4523.
Bolli N, Nicoletti I, De Marco MF, Bigerna B, Pucciarini A, Mannucci R et al. Born to be exported: COOH-terminal nuclear export signals of different strength ensure cytoplasmic accumulation of nucleophosmin leukemic mutants. Cancer Res 2007; 67: 6230–6237.
Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 2012; 120: 1765–1773.
Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res 2007; 13 (18 Pt 1): 5291–5294.
Chandra J . Oxidative stress by targeted agents promotes cytotoxicity in hematologic malignancies. Antioxid Redox Signal 2009; 11: 1123–1137.
McCloskey SM, McMullin MF, Walker B, Irvine AE . The therapeutic potential of the proteasome in leukaemia. Hematol Oncol 2008; 26: 73–81.
Fuchs O, Provaznikova D, Marinov I, Kuzelova K, Spicka I . Antiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cells. Cardiovasc Hematol Disord Drug Targets 2009; 9: 62–77.
Heaney NB, Pellicano F, Zhang B, Crawford L, Chu S, Kazmi SM et al. Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells. Blood 2010; 115: 2241–2250.
Ling YH, Liebes L, Zou Y, Perez-Soler R . Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem 2003; 278: 33714–33723.
Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D . The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 2006; 107: 257–264.
Hideshima T, Richardson PG, Anderson KC . Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther 2011; 10: 2034–2042.
Ohshima-Hosoyama S, Davare MA, Hosoyama T, Nelon LD, Keller C . Bortezomib stabilizes NOXA and triggers ROS-associated apoptosis in medulloblastoma. J Neurooncol 2011; 105: 475–483.
Pei XY, Dai Y, Grant S . The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia 2003; 17: 2036–2045.
Lioni M, Noma K, Snyder A, Klein-Szanto A, Diehl JA, Rustgi AK et al. Bortezomib induces apoptosis in esophageal squamous cell carcinoma cells through activation of the p38 mitogen-activated protein kinase pathway. Mol Cancer Ther 2008; 7: 2866–2875.
Zhu K, Dunner K Jr, McConkey DJ . Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene 2010; 29: 451–462.
Li C, Johnson DE . Bortezomib induces autophagy in head and neck squamous cell carcinoma cells via JNK activation. Cancer Lett 2012; 314: 102–107.
Selimovic D, Porzig BB, El-Khattouti A, Badura HE, Ahmad M, Ghanjati F et al. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal 2013; 25: 308–318.
Terpos E, Sezer O, Croucher P, Dimopoulos MA . Myeloma bone disease and proteasome inhibition therapies. Blood 2007; 110: 1098–1104.
Naumann I, Kappler R, von Schweinitz D, Debatin KM, Fulda S . Bortezomib primes neuroblastoma cells for TRAIL-induced apoptosis by linking the death receptor to the mitochondrial pathway. Clin Cancer Res 2011; 17: 3204–3218.
Lengfelder E, Hofmann WK, Nowak D . Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 2012; 26: 433–442.
Munshi NC, Tricot G, Desikan R, Badros A, Zangari M, Toor A et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia 2002; 16: 1835–1837.
Rousselot P, Larghero J, Arnulf B, Poupon J, Royer B, Tibi A et al. A clinical and pharmacological study of arsenic trioxide in advanced multiple myeloma patients. Leukemia 2004; 18: 1518–1521.
Amadori S, Fenaux P, Ludwig H, O'Dwyer M, Sanz M . Use of arsenic trioxide in haematological malignancies: insight into the clinical development of a novel agent. Curr Med Res Opin 2005; 21: 403–411.
Roboz GJ, Ritchie EK, Curcio T, Provenzano J, Carlin R, Samuel M et al. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer 2008; 113: 2504–2511.
Hole PS, Darley RL, Tonks A . Do reactive oxygen species play a role in myeloid leukemias? Blood 2011; 117: 5816–5826.
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S . Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 1999; 94: 2102–2111.
Chen YC, Lin-Shiau SY, Lin JK . Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol 1998; 177: 324–333.
Platanias LC . Biological responses to arsenic compounds. J Biol Chem 2009; 284: 18583–18587.
Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A, Macleod RA et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 2005; 19: 1760–1767.
Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ et al. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci 2009; 29: 6250–6265.
Huang M, Whang P, Chodaparambil JV, Pollyea DA, Kusler B, Xu L et al. Reactive oxygen species regulate nucleostemin oligomerization and protein degradation. J Biol Chem 2011; 286: 11035–11046.
Amir Y, Edward O-A, Utpal B . A protocol for in vivo detection of reactive oxygen species. Protoc Exch 2008, 1038 Available at http://www.nature.com/protocolexchange/protocols/414.
Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005; 106: 3733–3739.
Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005; 106: 3747–3754.
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746.
Suzuki T, Kiyoi H, Ozeki K, Tomita A, Yamaji S, Suzuki R et al. Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 2005; 106: 2854–2861.
Ren R . Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 2005; 5: 172–183.
Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH . Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood 2001; 98: 805–813.
Wang YE, Pernet O, Lee B . Regulation of the nucleocytoplasmic trafficking of viral and cellular proteins by ubiquitin and small ubiquitin-related modifiers. Biol Cell 2012; 104: 121–138.
Sekimoto T, Yoneda Y . Intrinsic and extrinsic negative regulators of nuclear protein transport processes. Genes Cells 2012; 17: 525–535.
Kodiha M, Stochaj U . Nuclear transport: a switch for the oxidative stress-signaling circuit? J Signal Transduct 2012; 2012: 208650.
Sadoul K, Wang J, Diagouraga B, Khochbin S . The tale of protein lysine acetylation in the cytoplasm. J Biomed Biotechnol 2011; 2011: 970382.
Shinmura K, Tarapore P, Tokuyama Y, George KR, Fukasawa K . Characterization of centrosomal association of nucleophosmin/B23 linked to Crm1 activity. FEBS Lett 2005; 579: 6621–6634.
Mariano AR, Colombo E, Luzi L, Martinelli P, Volorio S, Bernard L et al. Cytoplasmic localization of NPM in myeloid leukemias is dictated by gain-of-function mutations that create a functional nuclear export signal. Oncogene 2006; 25: 4376–4380.
Grummitt CG, Townsley FM, Johnson CM, Warren AJ, Bycroft M . Structural consequences of nucleophosmin mutations in acute myeloid leukemia. J Biol Chem 2008; 283: 23326–23332.
Fribley A, Zeng Q, Wang CY . Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol 2004; 24: 9695–9704.
Yu C, Rahmani M, Dent P, Grant S . The hierarchical relationship between MAPK signaling and ROS generation in human leukemia cells undergoing apoptosis in response to the proteasome inhibitor Bortezomib. Exp Cell Res 2004; 295: 555–566.
Lu J, Chew EH, Holmgren A . Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci USA 2007; 104: 12288–12293.
Flora SJ . Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 2011; 51: 257–281.
Wang J, Li L, Cang H, Shi G, Yi J . NADPH oxidase-derived reactive oxygen species are responsible for the high susceptibility to arsenic cytotoxicity in acute promyelocytic leukemia cells. Leuk Res 2008; 32: 429–436.
Tseng HY, Liu ZM, Huang HS . NADPH oxidase-produced superoxide mediates EGFR transactivation by c-Src in arsenic trioxide-stimulated human keratinocytes. Arch Toxicol 2012; 86: 935–945.
Kermani IA, Ghahremanfard F, Sanaat Z, Dolatkhah R . J Clin Diag Res 2011; 5: 1402–1405.
Wetzler M, Andrews C, Ford LA, Tighe S, Barcos M, Sait SN et al. Phase 1 study of arsenic trioxide, high-dose cytarabine, and idarubicin to down-regulate constitutive signal transducer and activator of transcription 3 activity in patients aged <60 years with acute myeloid leukemia. Cancer 2011; 117: 4861–4868.
Acknowledgements
This work was supported by a translational research grant from the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, M., Thomas, D., Li, M. et al. Role of cysteine 288 in nucleophosmin cytoplasmic mutations: sensitization to toxicity induced by arsenic trioxide and bortezomib. Leukemia 27, 1970–1980 (2013). https://doi.org/10.1038/leu.2013.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.222
Keywords
This article is cited by
-
Cystine uptake inhibition potentiates front-line therapies in acute myeloid leukemia
Leukemia (2022)
-
NSC348884 cytotoxicity is not mediated by inhibition of nucleophosmin oligomerization
Scientific Reports (2021)
-
Nucleophosmin: from structure and function to disease development
BMC Molecular Biology (2016)