Abstract
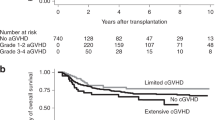
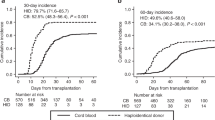
This retrospective report compared the results of graft source on outcome after allogeneic stem cell transplantation (allo-SCT) in patients with hematologic malignancies receiving a reduced intensity conditioning (RIC) regimen. A total of 152 patients received either a RIC allo-SCT using a 9/10 mismatched unrelated donor (MisMUD, n=42) or a double unrelated umbilical cord blood (dUCB, n=110) graft. With a median follow-up of 30.3 months, the cumulative incidence of non-relapse mortality was 26% in the dUCB group versus 24% in the MisMUD group (P=0.95). Grade 3–4 acute graft-versus-host disease (GVHD) incidence was 19.7% in the dUCB group versus 21.4% in the MisMUD group (P=0.83). The cumulative incidence of extensive chronic GVHD at 2 years was 6.4% in the dUCB group versus 21.4% in the MisMUD group (P=0.02). The Kaplan–Meier estimate of overall survival at 2 years was comparable between both groups (52.3% (95% confidence interval (CI), 42.1–61.5%) in the dUCB group versus 47.9% (95% CI, 31.6–62.4%) in the MisMUD group, P=0.55). Progression-free survival at 2 years was 43.3% (95% CI, 33.7–52.5%) in the dUCB group versus 38.3% (95% CI, 23.2–53.3%) in the MisMUD group (P=0.55). These data suggest that dUCB is a valid alternative graft source with significantly less chronic GVHD compared with MisMUD in the setting of RIC allo-SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004; 351: 2265–2275.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004; 351: 2276–2285.
Sanz MA, Sanz GF . Unrelated donor umbilical cord blood transplantation in adults. Leukemia 2002; 16: 1984–1991.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11: 653–660.
Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010; 116: 4693–4699.
Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood 2007; 110: 4606–4613.
Champlin R, Khouri I, Komblau S, Molidrem J, Giralt S . Reinventing bone marrow transplantation. Nonmyeloablative preparative regimens and induction of graft-vs-malignancy effect. Oncology (Williston Park) 1999; 13: 621–628, discussion 631, 635-628, 641.
Storb R . Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Pract Res Clin Haematol 2007; 20: 85–90.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia 2012; 26: 2462–2468.
Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007; 110: 3064–3070.
Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant 2012; 18: 805–812.
Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood 2012; 119: 5591–5598.
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol 2006; 24: 5695–5702.
Brissot E, Chevallier P, Guillaume T, Delaunay J, Ayari S, Dubruille V et al. Prophylaxis with mycophenolate mofetil and CsA can decrease the incidence of severe acute GVHD after antithymocyte globulin-based reduced-intensity preparative regimen and allo-SCT from HLA-matched unrelated donors. Bone Marrow Transplant 2010; 45: 786–788.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Malard F, Szydlo RM, Brissot E, Chevallier P, Guillaume T, Delaunay J et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16: 28–34.
Mohty M, Jacot W, Faucher C, Bay JO, Zandotti C, Collet L et al. Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia 2003; 17: 2168–2177.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood 2011; 117: 4651–4657.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant 2006; 12: 560–565.
Mohty M, Labopin M, Balere ML, Socie G, Milpied N, Tabrizi R et al. Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Leukemia 2010; 24: 1867–1874.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009; 10: 855–864.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 2011; 117: 6963–6970.
Cahu X, Rialland F, Touzeau C, Chevallier P, Guillaume T, Delaunay J et al. Infectious complications after unrelated umbilical cord blood transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant 2009; 15: 1531–1537.
Petersdorf EW, Gooley T, Malkki M, Horowitz M . Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens 2007; 69 (Suppl 1): 25–30.
Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2011; 17: 885–892.
Acknowledgements
We thank the nursing staff for providing excellent care for our patients, and the following physicians for their dedicated patient care: N Blin, A Clavert, V Dubruille, T Gastinne, B Mahe, F Mechinaud and F Rialland. MM thanks Pr JV de Melo (Adelaide, Australia) for critical reading of the manuscript. FM was supported by educational grants from the ‘Association for Training, Education and Research in Hematology, Immunology and Transplantation’ (ATERHIT). We also thank the ‘Région Pays de Loire’, the ‘Association pour la Recherche sur le Cancer (ARC; grant #3175 to MM and BG)’, the ‘Fondation de France’, the ‘Fondation contre la Leucémie’, the ‘Agence de Biomédecine’, the ‘Association Cent pour Sang la Vie’, the ‘Association Laurette Fuguain’ and the IRGHET for their generous and continuous support for our clinical and basic research work. Our group is supported by several grants from the French national cancer institute (PHRC, INCa to MM). We acknowledge the continuous support of the cell banking facility (‘tumurotheque’) of the CHU de Nantes.
Author contributions
F Malard: collected, assembled and analyzed data, performed statistical analysis and wrote the manuscript; S Fürst and M Loirat: collected data and commented on the manuscript; P Chevallier, T Guillaume, J Delaunay, Jean El-Cheikh, S. Le Gouill, P Moreau and Didier Blaise: recruited patients and commented on the manuscript; M Mohty: recruited patients, supervised research, analyzed data and wrote the manuscript; all authors approved submission of the manuscript for publication purposes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malard, F., Fürst, S., Loirat, M. et al. Effect of graft source on mismatched unrelated donor hemopoietic stem cell transplantation after reduced intensity conditioning. Leukemia 27, 2113–2117 (2013). https://doi.org/10.1038/leu.2013.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.170