Abstract
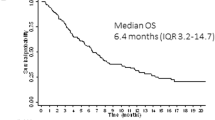
The mTORC1 signaling pathway is constitutively activated in almost all acute myelogenous leukemia (AML) patients. We conducted a phase Ib trial combining RAD001 (everolimus), an allosteric inhibitor of mTORC1, and conventional chemotherapy, in AML patients under 65 years of age at first relapse (clinical trial NCT 01074086). Increasing doses of RAD001 from 10–70 mg were administrated orally on days 1 and 7 (d1 and d7) of a 3+7 daunorubicin+cytarabine conventional induction chemotherapy regimen. Twenty-eight patients were enrolled in this trial. The treatment was well tolerated with <10% toxicity, mainly involving the gastrointestinal tract and lungs. In this phase Ib trial, the RAD001 maximum tolerated dose was not reached at 70 mg. Sixty-eight percent of patients achieved CR, of which 14 received a double induction. Eight subsequently were intensified with allogeneic-stem cell transplant. Strong plasma inhibition of P-p70S6K was observed after RAD001 administration, still detectable at d7 (d7)at the 70 mg dosage. CR rates in patients with RAD001 areas under or above the curve median were 53% versus 85%. A 70 mg dose of RAD001 at d1 and d7 of an induction chemotherapy regimen for AML has acceptable toxicity and may improve treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smith ML, Hills RK, Grimwade D . Independent prognostic variables in acute myeloid leukaemia. Blood Rev 2011; 25: 39–51.
Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem 2007; 14: 2009–2023.
Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 2005; 105: 2527–2534.
Recher C, Dos Santos C, Demur C, Payrastre B . mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle 2005; 4: 1540–1549.
Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood 2008; 111: 379–382.
Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M . Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 2003; 102: 972–980.
Xu Q, Thompson JE, Carroll M . mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood 2005; 106: 4261–4268.
Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res 2009; 15: 6732–6739.
Amadori S, Stasi R, Martelli AM, Venditti A, Meloni G, Pane F et al. Temsirolimus, an mTOR inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with acute myeloid leukaemia: results of a phase II GIMEMA study (AML-1107). Br J Haematol 2012; 156: 205–212.
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008; 26: 1603–1610.
O'Donnell A, Faivre S, Burris HA, Rea D, Papadimitrakopoulou V, Shand N et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008; 26: 1588–1595.
Chevallier P, Fornecker L, Lioure B, Bene MC, Pigneux A, Recher C et al. Tandem versus single autologous peripheral blood stem cell transplantation as post-remission therapy in adult acute myeloid leukemia patients under 60 in first complete remission: results of the multicenter prospective phase III GOELAMS LAM-2001 trial. Leukemia 2010; 24: 1380–1385.
Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 2003; 101: 64–70.
Heil G, Krauter J, Raghavachar A, Bergmann L, Hoelzer D, Fiedler W et al. Risk-adapted induction and consolidation therapy in adults with de novo AML aged </=60 years: results of a prospective multicenter trial. Ann Hematol 2004; 83: 336–344.
Goirand F, Royer B, Hulin A, Saint-Marcoux F . level of evidence for therapeutic drug monitoring of everolimus. Therapie 2011; 66: 57–61.
Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood 2006; 108: 3477–3483.
Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A et al. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res 2010; 16: 5424–5435.
Martin Ramos ML, Lopez Pastor M, de la Serna Torroba J, Ayala R, Garcia Alonso L, Barreiro Miranda E . (Cytogenetic risk categories in acute myeloid leukemia: a comparison between MRC (Medical Research Council) and SWOG (Southwest Oncology Group) models)). Med Clin (Barc) 2003; 121: 121–125.
O'Reilly T, McSheehy PM, Wartmann M, Lassota P, Brandt R, Lane HA . Evaluation of the mTOR inhibitor, everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anticancer Drugs 2011; 22: 58–78.
Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol 2009; 27: 4536–4541.
Estey EH, Keating MJ, McCredie KB, Bodey GP, Freireich EJ . Causes of initial remission induction failure in acute myelogenous leukemia. Blood 1982; 60: 309–315.
Schwartz RS, Mackintosh FR, Schrier SL, Greenberg PL . Multivariate analysis of factors associated with invasive fungal disease during remission induction therapy for acute myelogenous leukemia. Cancer 1984; 53: 411–419.
Freund M, Link H, Diedrich H, LeBlanc S, Wilke HJ, Poliwoda H . High-dose ara-C and etoposide in refractory or relapsing acute leukemia. Cancer Chemother Pharmacol 1991; 28: 487–490.
Bow EJ, Kilpatrick MG, Scott BA, Clinch JJ, Cheang MS . Acute myeloid leukemia in Manitoba. The consequences of standard "7+3" remission-induction therapy followed by high dose cytarabine postremission consolidation for myelosuppression, infectious morbidity, and outcome. Cancer 1994; 74: 52–60.
Kern W, Schleyer E, Unterhalt M, Wormann B, Buchner T, Hiddemann W . High antileukemic activity of sequential high dose cytosine arabinoside and mitoxantrone in patients with refractory acute leukemias. Results of a clinical phase II study. Cancer 1997; 79: 59–68.
Faderl S, Verstovsek S, Cortes J, Ravandi F, Beran M, Garcia-Manero G et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood 2006; 108: 45–51.
Estey EH . Growth factors in acute myeloid leukaemia. Best Pract Res Clin Haematol 2001; 14: 175–187.
Monma F, Katayama N . (Management of microbial infections in myeloid leukemia). Nihon Rinsho 2009; 67: 1969–1973.
Usuki K, Urabe A, Masaoka T, Ohno R, Mizoguchi H, Hamajima N et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol 2002; 116: 103–112.
Perl AE, Kasner MT, Shank D, Luger SM, Carroll M . Single-cell pharmacodynamic monitoring of S6 ribosomal protein phosphorylation in AML blasts during a clinical trial combining the mTOR inhibitor sirolimus and intensive chemotherapy. Clin Cancer Res 2012; 18: 1716–1725.
Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res 2012; 72: 6468–6476.
Archimbaud E, Leblond V, Michallet M, Cordonnier C, Fenaux P, Travade P et al. Intensive sequential chemotherapy with mitoxantrone and continuous infusion etoposide and cytarabine for previously treated acute myelogenous leukemia. Blood 1991; 77: 1894–1900.
Brincker H, Christensen BE . Long-term survival and late relapses in acute leukaemia in adults. Br J Haematol 1990; 74: 156–160.
Norkin M, Uberti JP, Schiffer CA . Very late recurrences of leukemia: why does leukemia awake after many years of dormancy? Leuk Res 2011; 35: 139–144.
Verma D, Kantarjian H, Faderl S, O'Brien S, Pierce S, Vu K et al. Late relapses in acute myeloid leukemia: analysis of characteristics and outcome. Leuk Lymphoma 2010; 51: 778–782.
Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 2011; 117: 3294–3301.
Chen W, Drakos E, Grammatikakis I, Schlette EJ, Li J, Leventaki V et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol Cancer 2010; 9: 292.
Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia 2012; 26: 1195–1202.
Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 2009; 114: 1618–1627.
Acknowledgements
We thank all the GOELAMS investigators, the molecular biologists, Laurence Lode, Olivier Kosmider, Odile Blanchet, Marie–Pierre Gaub and clinical trial office staff in Tours and Novartis.
Author contributions
Conception and design: Sophie Park, Didier Bouscary, Christian Recher, Annabelle Merlat. Administrative support: Roselyne Delepine, François Dreyfus, Catherine Lacombe and Patrick Mayeux. Provision of study materials or patients: Sophie Park, Christian Recher, Norbert Vey, Thomas Prebet, Patrice Chevallier, Jean-Yves Cahn, Thibault Leguay, Pierre Boris, Francis Witz, Thierry Lamy, Jérôme Tamburini, Annabelle Merlat, Marie-Christine Béné, Norbert Ifrah and Didier Bouscary. Collection and assembly of data: Roselyne Delepine. Data analysis and interpretation: Sophie Park, Nicolas Chapuis, Franck Saint Marcoux, Christian Recher, Norbert Vey, Thomas Prebet, Patrice Chevallier,Jean-Yves Cahn, Thibault Leguay, Pierre Boris, Francis Witz, Thierry Lamy, Annabelle Merlat, Roselyne Delepine, Francois Dreyfus, Marie-Christine Béné, Norbert Ifrah and Didier Bouscary.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
AM is employed at BU oncologie Novartis Pharma SAS, Novartis, Rueil-Malmaison, France. The rest of the authors declare no conflict of interest.
Additional information
Oral communication at the 53th Annual Meeting of the American Society of Hematology, San Diego, 2011, Blood, 118: Abstract 945. Oral communication at the Annual Meeting of the Société Française d’Hématologie, Paris, 2012. Oral communication at the Annual Meeting of the European Society of Hematology, Amsterdam, 2012, Haematologica, 2012, 97 s1: 469, Abstract 1136.
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Park, S., Chapuis, N., Saint Marcoux, F. et al. A phase Ib GOELAMS study of the mTOR inhibitor RAD001 in association with chemotherapy for AML patients in first relapse. Leukemia 27, 1479–1486 (2013). https://doi.org/10.1038/leu.2013.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.17
Keywords
This article is cited by
-
The dual role of autophagy in acute myeloid leukemia
Journal of Hematology & Oncology (2022)
-
Memantine potentiates cytarabine-induced cell death of acute leukemia correlating with inhibition of Kv1.3 potassium channels, AKT and ERK1/2 signaling
Cell Communication and Signaling (2019)
-
Sirolimus enhances remission induction in patients with high risk acute myeloid leukemia and mTORC1 target inhibition
Investigational New Drugs (2018)
-
Understanding of leukemic stem cells and their clinical implications
Molecular Cancer (2017)
-
The PI3K/mTOR dual inhibitor BEZ235 suppresses proliferation and migration and reverses multidrug resistance in acute myeloid leukemia
Acta Pharmacologica Sinica (2017)