Abstract
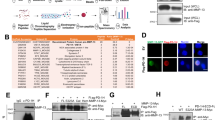
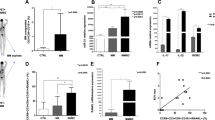
The key nuclear export protein CRM1/XPO1 may represent a promising novel therapeutic target in human multiple myeloma (MM). Here we showed that chromosome region maintenance 1 (CRM1) is highly expressed in patients with MM, plasma cell leukemia cells and increased in patient cells resistant to bortezomib treatment. CRM1 expression also correlates with increased lytic bone and shorter survival. Importantly, CRM1 knockdown inhibits MM cell viability. Novel, oral, irreversible selective inhibitors of nuclear export (SINEs) targeting CRM1 (KPT-185, KPT-330) induce cytotoxicity against MM cells (ED50<200 nM), alone and cocultured with bone marrow stromal cells (BMSCs) or osteoclasts (OC). SINEs trigger nuclear accumulation of multiple CRM1 cargo tumor suppressor proteins followed by growth arrest and apoptosis in MM cells. They further block c-myc, Mcl-1, and nuclear factor κB (NF-κB) activity. SINEs induce proteasome-dependent CRM1 protein degradation; concurrently, they upregulate CRM1, p53-targeted, apoptosis-related, anti-inflammatory and stress-related gene transcripts in MM cells. In SCID mice with diffuse human MM bone lesions, SINEs show strong anti-MM activity, inhibit MM-induced bone lysis and prolong survival. Moreover, SINEs directly impair osteoclastogenesis and bone resorption via blockade of RANKL-induced NF-κB and NFATc1, with minimal impact on osteoblasts and BMSCs. These results support clinical development of SINE CRM1 antagonists to improve patient outcome in MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xu D, Grishin NV, Chook YM . NESdb: a database of NES-containing CRM1 cargos. Mol Biol Cell 2012; 23: 3673–3676.
Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M et al. Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol 2007; 9: 1175–1183.
Shao C, Lu C, Chen L, Koty PP, Cobos E, Gao W . p53-Dependent anticancer effects of leptomycin B on lung adenocarcinoma. Cancer Chemother Pharmacol 2011; 67: 1369–1380.
Turner JG, Dawson J, Sullivan DM . Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol 2012; 83: 1021–1032.
Vogt PK, Jiang H, Aoki M . Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 2005; 4: 908–913.
Senapedis WT, Kennedy CJ, Boyle PM, Silver PA . Whole genome siRNA cell-based screen links mitochondria to Akt signaling network through uncoupling of electron transport chain. Mol Biol Cell 2011; 22: 1791–1805.
Huang TT, Kudo N, Yoshida M, Miyamoto S . A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA 2000; 97: 1014–1019.
Brodie KM, Henderson BR . Characterization of BRCA1 protein targeting, dynamics, and function at the centrosome: a role for the nuclear export signal, CRM1, and Aurora A kinase. J Biol Chem 2012; 287: 7701–7716.
Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P et al. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep 2009; 21: 229–235.
Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ . Prognostic value of CRM1 in pancreas cancer. Clin Invest Med 2009; 32: E315.
Noske A, Weichert W, Niesporek S, Roske A, Buckendahl AC, Koch I et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer 2008; 112: 1733–1743.
van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D et al. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer 2009; 124: 1829–1840.
Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011; 475: 101–105.
Balatti V, Bottoni A, Palamarchuk A, Alder H, Rassenti LZ, Kipps TJ et al. NOTCH1 mutations in CLL associated with trisomy 12. Blood 2012; 119: 329–331.
Turner JG, Engel R, Derderian JA, Jove R, Sullivan DM . Human topoisomerase IIalpha nuclear export is mediated by two CRM-1-dependent nuclear export signals. J Cell Sci 2004; 117: 3061–3071.
Turner JG, Sullivan DM . CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem 2008; 15: 2648–2655.
Turner JG, Marchion DC, Dawson JL, Emmons MF, Hazlehurst LA, Washausen P et al. Human multiple myeloma cells are sensitized to topoisomerase II inhibitors by CRM1 inhibition. Cancer Res 2009; 69: 6899–6905.
Newlands ES, Rustin GJ, Brampton MH . Phase I trial of elactocin. Br J Cancer 1996; 74: 648–649.
Sakakibara K, Saito N, Sato T, Suzuki A, Hasegawa Y, Friedman JM et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood 2011; 118: 3922–3931.
Etchin J, Sun Q, Kentsis A, Farmer A, Zhang ZC, Sanda T et al. Anti-leukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 2012; 27: 66–74.
Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 2012; 120: 4621–4634.
Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 2012; 120: 1765–1773.
Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood 2012; 120: 1877–1887.
Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF et al. Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood 2007; 110: 1656–1663.
Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112: 1329–1337.
Zhan F, Barlogie B, Arzoumanian V, Huang Y, Williams DR, Hollmig K et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood 2007; 109: 1692–1700.
Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S et al. The molecular classification of multiple myeloma. Blood 2006; 108: 2020–2028.
Shaughnessy JD Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2007; 109: 2276–2284.
Gutierrez NC, Ocio EM, de Las Rivas J, Maiso P, Delgado M, Ferminan E et al. Gene expression profiling of B lymphocytes and plasma cells from Waldenstrom’s macroglobulinemia: comparison with expression patterns of the same cell counterparts from chronic lymphocytic leukemia, multiple myeloma and normal individuals. Leukemia 2007; 21: 541–549.
Mattioli M, Agnelli L, Fabris S, Baldini L, Morabito F, Bicciato S et al. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct IGH translocations in multiple myeloma. Oncogene 2005; 24: 2461–2473.
Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood 2007; 109: 3177–3188.
Quinn J, Glassford J, Percy L, Munson P, Marafioti T, Rodriguez-Justo M et al. APRIL promotes cell-cycle progression in primary multiple myeloma cells: influence of D-type cyclin group and translocation status. Blood 2011; 117: 890–901.
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006; 66: 6675–6682.
Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004; 103: 3148–3157.
McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med 2010; 16: 483–489.
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002; 3: 889–901.
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 2005; 202: 345–351.
Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 2004; 200: 941–946.
Tiedemann RE, Zhu YX, Schmidt J, Shi CX, Sereduk C, Yin H et al. Identification of molecular vulnerabilities in human multiple myeloma cells by RNA interference lethality screening of the druggable genome. Cancer Res 2012; 72: 757–768.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328.
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 2006; 203: 1657–1663.
Grinberg AV, Hu CD, Kerppola TK . Visualization of Myc/Max/Mad family dimers and the competition for dimerization in living cells. Mol Cell Biol 2004; 24: 4294–4308.
Golomb L, Bublik DR, Wilder S, Nevo R, Kiss V, Grabusic K et al. Importin 7 and exportin 1 link c-Myc and p53 to regulation of ribosomal biogenesis. Mol Cell 2012; 45: 222–232.
van der Watt PJ, Leaner VD . The nuclear exporter, Crm1, is regulated by NFY and Sp1 in cancer cells and repressed by p53 in response to DNA damage. Biochim Biophys Acta 2011; 1809: 316–326.
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC . Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 2007; 7: 585–598.
Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PB et al. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res 2009; 69: 510–517.
Acknowledgements
We are grateful for Hao Wang, Matt Ma, Michelle Chen, Yiguo Hu and Sunyoung Kong for excellent technical support to the project. We thank the input from Haoqiang Ying and Dilara McCauley from Karyopharm Pharmaceutics. We also thank the nursing staff and clinical research coordinators of the LeBow Institute for Myeloma Therapeutics and the Jerome Lipper Multiple Myeloma Center of the Dana-Farber Cancer Institute for support and help in providing primary tumor specimens for this study. This study was supported by National Institutes of Health Grants RO-1 50947, PO1–78378 and DF/HCC SPORE in Multiple Myeloma P50CA100707; and the Lebow Fund to Cure Myeloma (KCA); KCA is an American Cancer Society Clinical Research Professor.
Author Contributions
Y-TT, YL, SS, MK, KCA conceptualized research and formed the hypothesis of this paper; Y-TT, YL, YC, SS, ALK designed, performed experiments, analyzed data; FZ, AC, AAM analyzed expression data; CA, MC, YC, MYZ, AC, MR, DT, J-RS-M, TK, YG, WS, IG, LW, performed the in vitro research and collected data; SS, ALK, CA, MC, MYZ designed, performed and analyzed animal work; YL, YC, SS, MK, LW, SAS provided reagents, analytic tools and input to studies; NCM, PR, KCA provided MM patient samples; Y-TT wrote the manuscript; Y-TT, SS, MK, KCA critically evaluated and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
YL, WS, J-RS-M, TK, SS, MK are employees of Karyopharm Therapeutics Inc., whose product was used in this research PR serves on advisory boards to Millennium, Celgene, Novartis, Johnson & Johnson and Bristol-Myers Squibb NCM serves on advisory boards to Millennium, Celgene and Novartis KCA serves on advisory boards to Onyx, Celgene, Gilead and Sanofi-Aventis. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Tai, YT., Landesman, Y., Acharya, C. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014). https://doi.org/10.1038/leu.2013.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.115
Keywords
This article is cited by
-
Targeting TRIP13 in favorable histology Wilms tumor with nuclear export inhibitors synergizes with doxorubicin
Communications Biology (2024)
-
The synergy of the XPO1 inhibitors combined with the BET inhibitor INCB057643 in high-grade B-cell lymphoma via downregulation of MYC expression
Scientific Reports (2023)
-
Disruption to the FOXO-PRDM1 axis resulting from deletions of chromosome 6 in acute lymphoblastic leukaemia
Leukemia (2023)
-
Selinexor–Bortezomib–Dexamethasone: A Review in Previously Treated Multiple Myeloma
Targeted Oncology (2023)
-
Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t(11;14)
npj Precision Oncology (2022)