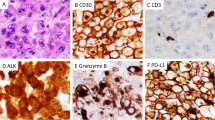
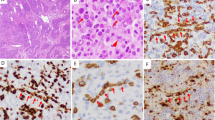
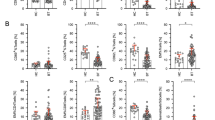
Abstract
We studied the prognostic value of minimal disseminated disease (MDD) and anti-ALK immune response in children with NPM-ALK-positive anaplastic-large cell lymphoma (ALCL) and evaluated their potential for risk stratification. NPM-ALK transcripts were analyzed by RT-PCR in bone marrow/peripheral blood of 128 ALCL patients at diagnosis, whereas ALK antibody titers in plasma were assessed using an immunocytochemical approach. MDD was positive in 59% of patients and 96% showed an anti-ALK response. Using MDD and antibody titer results, patients could be divided into three biological risk groups (bRG) with different prognosis: high risk (bHR): MDD-positive and antibody titer ⩽1/750, 26/128 (20%); low risk (bLR): MDD negative and antibody titer >1/750, 40/128 (31%); intermediate risk (bIR): all remaining patients, 62/128 (48%). Progression-free survival was 28% (s.e., 9%), 68% (s.e., 6%) and 93% (s.e., 4%) for bHR, bIR and bLR, respectively (P<0.0001). Survival was 71% (s.e., 9%), 83% (s.e., 5%) and 98% (s.e., 2%) for bHR, bIR and bLR (P=0.02). Only bHR and histology other than common type were predictive of higher risk of failure (hazard ratio 4.9 and 2.7, respectively) in multivariate analysis. Stratification of ALCL patients based on MDD and anti-ALK titer should be considered in future ALCL trials to optimize treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol 2005; 131: 39–49.
Delsol G . The 2008 WHO lymphoma classification. Ann Pathol 2008; 1: S20–S24.
Brugieres L, Deley MC, Pacquement H, Meguerian-Bedoyan Z, Terrier-Lacombe MJ, Robert A et al. CD30(+) anaplastic large-cell lymphoma in children: analysis of 82 patients enrolled in two consecutive studies of the French Society of Pediatric Oncology. Blood 1998; 92: 3591–3598.
Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 2001; 97: 3699–3706.
Brugieres L, Pacquement H, Le Deley MC, Leverger G, Lutz P, Paillard C et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol 2009; 27: 5056–5061.
Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood 2008; 111: 1560–1566.
Mussolin L, Pillon M, d’Amore ES, Santoro N, Lombardi A, Fagioli F et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia 2005; 19: 1643–1647.
Lamant L, McCarthy K, d′Amore E, Klapper W, Nakagawa A, Fraga M et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol 2011; 29: 4669–4676.
Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood 2007; 110: 670–677.
Pulford K, Falini B, Banham AH, Codrington D, Roberton H, Hatton C et al. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood 2000; 96: 1605–1607.
Mussolin L, Bonvini P, Ait-Tahar K, Pillon M, Tridello G, Buffardi S et al. Kinetics of humoral response to ALK and its relationship with minimal residual disease in pediatric ALCL. Leukemia 2009; 23: 400–402.
Ait-Tahar K, Damm-Welk C, Burkhardt B, Zimmermann M, Klapper W, Reiter A et al. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 2010; 115: 3314–3319.
Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol 2009; 27: 897–903.
Kaplan E . Non-parametric estimation from incomplete observations. Am Stat Assos 1958; 53: 457–481.
Pillon M, Gregucci F, Lombardi A, Santoro N, Piglione M, Sala A et al. Results of AIEOP LNH-97 protocol for the treatment of anaplastic large cell lymphoma of childhood. Pediatr Blood Cancer 2012; e-pub ahead of print 2 March 2012; doi: 10.1002/pbc.24125.
Cox D . Regression models and life tables. J R Stat Soc 1972; 34: 187–220.
Lamant L, de Reynies A, Duplantier MM, Rickman DS, Sabourdy F, Giuriato S et al. Gene-expression profiling of systemic anaplastic large-cell lymphoma reveals differences based on ALK status and two distinct morphologic ALK+ subtypes. Blood 2007; 109: 2156–2164.
Ait-Tahar K, Barnardo M, Pulford K . CD4 T-Helper Responses to the Anaplastic Lymphoma kinase (ALK) protein in patients with ALK-positive Anaplastic Large-Cell Lymphoma. Cancer Res 2007; 67: 1898–1901.
Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci USA 2008; 105: 20852–20857.
Rimsza LM, Roberts RA, Miller TP, Unger JM, LeBlanc M, Braziel RM et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 2004; 103: 4251–4258.
Cannella S, Santoro A, Bruno G, Pillon M, Mussolin L, Mangili G et al. Germline mutations of the perforin gene are a frequent occurrence in childhood anaplastic large cell lymphoma. Cancer 2007; 109: 2566–2571.
Acknowledgements
The study was supported by Fondazione Città Della Speranza, Associazione Italiana contro le Leucemie (AIL) and by Camera di Commercio di Venezia. The study was also supported by a grant from the Deutsche Jose Carreras Leukämie-Stiftung (DJCLS08/09) to WW. CDW and WW are additionally supported by the Forschungshilfe peiper. KP was supported by Leukemia and Lymphoma research, the Sam Foye Fund, Julian Starmer-Smith Lymphoma fund and the National Institute for Health Research Biomedical Research Center Program. We are grateful to Dr Elisa Carraro for data management. We thank the Colleagues from various AIEOP and BFM centers for contributing biological samples and patient clinical information.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Mussolin, L., Damm-Welk, C., Pillon, M. et al. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia 27, 416–422 (2013). https://doi.org/10.1038/leu.2012.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.205