Abstract
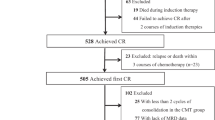
We conducted a systemic evaluation to describe the effect of minimal residual disease (MRD) kinetics on long-term allogeneic transplantation outcome by analyzing 95 adult transplants with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-positive ALL) who received first-line two courses of imatinib-based chemotherapy (median follow-up 5 years). MRD monitoring was centrally evaluated by real-time quantitative PCR (4.5 log sensitivity). After the first course of imatinib-based chemotherapy, 33 patients (34.7%) achieved at least major molecular response. On the basis of MRD kinetics by the end of two courses of imatinib-based chemotherapy, we stratified entire patients into four subgroups: early-stable molecular responders (EMRs, n=33), late molecular responders (LMRs, n=35), intermediate molecular responders (IMRs, n=9) and poor molecular responders (PMRs, n=18). Multivariate analysis showed that the most powerful factor affecting long-term transplantation outcome was MRD kinetics. Compared with EMRs, IMRs or PMRs had significantly higher risk of treatment failure in terms of relapse and disease-free survival (DFS). LMRs had a tendency toward a lower DFS. Quantitative monitoring of MRD kinetics during the first-line imatinib-based chemotherapy course is useful in identifying subgroups of Ph-positive ALL transplants at a high risk of relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas DA, Faderl S, Cortes J, O’Brien S, Giles FJ, Kornblau SM et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 2004; 103: 4396–4407.
Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 2006; 24: 460–466.
Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 2006; 108: 1469–1477.
de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood 2007; 109: 1408–1413.
Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol 2010; 28: 3644–3652.
Ribera JM, Oriol A, González M, Vidriales B, Brunet S, Esteve J et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica 2010; 95: 87–95.
Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F et al. Karyotype at diagnosis is the major prognostic factor predicting relapse-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Haematologica 2008; 93: 287–290.
Jaso J, Thomas DA, Cunningham K, Jorgensen JL, Kantarjian HM, Medeiros LJ et al. Prognostic significance of immunophenotypic and karyotypic features of Philadelphia positive B-lymphoblastic leukemia in the era of tyrosine kinase inhibitors. Cancer 2011; 117: 4009–4017.
Preudhomme C, Henic N, Cazin B, Lai JL, Bertheas MF, Vanrumbeke M et al. Good correlation between RT-PCR analysis and relapse in Philadelphia (Ph1)-positive acute lymphoblastic leukemia (ALL). Leukemia 1997; 11: 294–298.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the prospective multicenter LALA-94 trial. Blood 2002; 100: 2357–2366.
Pane F, Cimino G, Izzo B, Camera A, Vitale A, Quintarelli C et al. Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult Philadelphia-positive acute lymphoblastic leukemia. Leukemia 2005; 19: 628–635.
Lee S, Kim DW, Kim YJ, Chung NG, Kim YL, Hwang JY et al. Minimal residual disease-based role of imatinib as a first-line interim therapy prior to allogeneic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2003; 102: 3068–3070.
Lee S, Kim YJ, Min CK, Kim HJ, Eom KS, Kim DW et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2005; 105: 3449–3457.
Lee S, Kim YJ, Chung NG, Lim J, Lee DG, Kim HJ et al. The extent of minimal residual disease reduction after the first 4-week imatinib therapy determines outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2009; 115: 561–570.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108: 28–37.
Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia 2006; 20: 1925–1930.
Lee S, Cho BS, Kim SY, Choi SM, Lee DG, Eom KS et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007; 13: 1083–1094.
Lee S, Chung NG, Cho BS, Eom KS, Kim YJ, Kim HJ et al. Donor-specific differences in long-term outcomes of myeloablative transplantation in adults with Philadelphia-negative acute lymphoblastic leukemia. Leukemia 2010; 24: 2110–2119.
Cho BS, Lee S, Kim YJ, Chung NG, Eom KS, Kim HJ et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia 2009; 23: 1763–1770.
Lee S, Kim DW, Cho B, Kim YJ, Kim YL, Hwang JY et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br J Haematol 2003; 120: 145–153.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Lee SJ, Vogelsang G, Flowers ME . Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003; 9: 215–233.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Gray RJ . A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Fine JP, Gray RJ . A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 456–509.
Yanada M, Sugiura I, Takeuchi J, Akiyama H, Maruta A, Ueda Y et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol 2008; 143: 503–510.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia 2003; 17: 2318–2357.
Branford S, Fletcher L, Cross NC, Müller MC, Hochhaus A, Kim DW et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008; 112: 3330–3338.
Müller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia 2009; 23: 1957–1963.
Pfeifer H, Wassmann B, Pavlova A, Wunderle L, Oldenburg J, Binckebanck A et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 2007; 110: 727–734.
Soverini S, Vitale A, Poerio A, Gnani A, Colarossi S, Iacobucci I et al. Philadelphia-positive acute lymphoblastic leukemia patients already harbor BCR-ABL kinase domain mutations at low levels at the time of diagnosis. Haematologica 2011; 96: 552–557.
Acknowledgements
This research was supported by Seoul St Mary’s Clinical Medicine Research Program in the year of 2009 and 2010, respectively, through the Catholic University of Korea. Statistical analyses performed in this article were advised by the Catholic Medical Center Clinical Research Coordinating Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, S., Kim, DW., Cho, BS. et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 26, 2367–2374 (2012). https://doi.org/10.1038/leu.2012.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.164
Keywords
This article is cited by
-
Minimal Residual Disease in the Management of B-Cell Acute Lymphoblastic Leukemia: A Systematic Review of Studies from Indian Settings
Indian Journal of Hematology and Blood Transfusion (2024)
-
MRD in ALL: Optimization and Innovations
Current Hematologic Malignancy Reports (2022)
-
Impact of donor type on long-term graft-versus-host disease-free/relapse-free survival for adult acute lymphoblastic leukemia in first remission
Bone Marrow Transplantation (2021)
-
Non-inferior long-term outcomes of adults with Philadelphia chromosome-like acute lymphoblastic leukemia
Bone Marrow Transplantation (2021)
-
Association between measurable residual disease kinetics and outcomes of Philadelphia chromosome-positive acute lymphoblastic leukemia
Annals of Hematology (2021)