Abstract
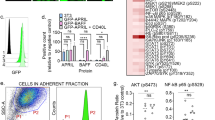
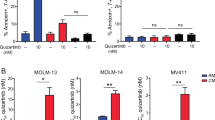
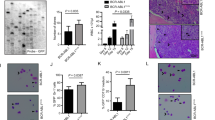
We and others have previously demonstrated that p210 Bcr-Abl tyrosine kinase inhibits stromal cell-derived factor-1α/CXCR4 chemokine receptor signaling, contributing to the deficient adhesion of chronic myeloid leukemia (CML) cells to bone marrow stroma. Conversely, exposure of CML cells to a tyrosine kinase inhibitor (TKI) enhances migration of CML cells towards stromal cell layers and promotes non-pharmacological resistance to imatinib. Src-related kinase Lyn is known to interact with CXCL12/CXCR4 signaling and is directly activated by p210 Bcr-Abl. In this study, we demonstrate that TKI treatment promoted CXCR4 redistribution into the lipid raft fraction, in which it co-localized with active phosphorylated form of Lyn (LynTyr396) in CML cells. Lyn inhibition or cholesterol depletion abrogated imatinib-induced migration, and dual Src/Abl kinase inhibitor dasatinib induced fewer CML cells to migrate to the stroma. These findings demonstrate the novel mechanism of microenvironment-mediated resistance through lipid raft modulation, which involves compartmental changes of the multivalent CXCR4 and Lyn complex. We propose that pharmacological targeting of lipid rafts may eliminate bone marrow-resident CML cells through interference with microenvironment-mediated resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rowley JD . A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and giemsa staining. Nature 1973; 243: 290–293.
Holyoake DT . Recent advances in the molecular and cellular biology of chronic myeloid leukaemia: lessons to be learned from the laboratory. Br J Haematol 2001; 113: 11–23.
Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061.
Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL . Punish the parent not the progeny. Blood 2005; 105: 1862–1866.
Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007; 109: 58–60.
Holtz MS, Forman SJ, Bhatia R . Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia 2005; 19: 1034–1041.
Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 1998; 273: 23169–23173.
Lataillade JJ, Clay D, Bourin P, Hérodin F, Dupuy C, Jasmin C et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: evidence for an autocrine/paracrine mechanism. Blood 2002; 99: 1117–1129.
Kim CH, Broxmeyer HE . In vitro behaviour of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow microenvironment. Blood 1998; 91: 100–110.
Geay JF, Buet D, Zhang Y, Foudi A, Jarrier P, Berthebaud M et al. P210BCR/ABL inhibits SDF-1 chemotactic response via alteration of CXCR4 signaling and down-regulation of CXCR4 expression. Cancer Res 2005; 65: 2676–2683.
Fei F, Stoddart S, Müschen M, Kim YM, Groffen J, Heisterkamp N . Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia. Leukemia 2010; 24: 813–820.
Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther 2008; 7: 48–58.
Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N . Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia 2011; 25: 1314–1323.
Chen YY, Malik M, Tomkowicz BE, Collman RG, Ptasznik A . BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells. Blood 2008; 111: 5182–5186.
Nakata Y, Tomkowicz B, Gewirtz AM, Ptasznik A . Integrin inhibition through Lyn-dependent cross talk from CXCR4 chemokine receptors in normal human CD34+ marrow cells. Blood 2006; 107: 4234–4239.
Ptasznik A, Urbanowska E, Chinta S, Costa MA, Katz BA, Stanislaus MA et al. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med 2002; 196: 667–678.
Sotirellis N, Johnson TM, Hibbs ML, Stanley IJ, Stanley E, Dunn AR et al. Autophosphorylation induces autoactivation and a decrease in the Src homology 2 domain accessibility of the Lyn protein kinase. J Biol Chem 1995; 270: 29773–29780.
Donella-Deana A, Cesaro L, Ruzzene M, Brunati AM, Marin O, Pinna LA . Spontaneous autophosphorylation of Lyn tyrosine kinase at both its activation segment and C-terminal tail confers altered substrate specificity. Biochemistry 1998; 37: 1438–1446.
Honda Z, Suzuki T, Hirose N, Aihara M, Shimizu T, Nada S et al. Roles of C-terminal Src kinase in the initiation and the termination of the high affinity IgE receptor-mediated signaling. J Biol Chem 1997; 272: 25753–25760.
Yoshizaki F, Nakayama H, Iwahara C, Takamori K, Ogawa H, Iwabuchi K . Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochim Biophys Acta 2008; 1780: 383–392.
Beran M, Pisa P, O′Brien S, Kurzrock R, Siciliano M, Cork A et al. Biological properties and growth in SCID mice of a new myelogenous leukemia cell line (KBM-5) derived from chronic myelogenous leukemia cells in the blastic phase. Cancer Res 1993; 53: 3603–3610.
Sawyers CL, McLaughlin J, Witte ON . Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995; 181: 307–313.
Tabe Y, Konopleva M, Munsell MF, Marini FC, Zompetta C, McQueen T et al. PML-RARα is associated with leptin-receptor induction: the role of mesenchymal stem cell–derived adipocytes in APL cell survival. Blood 2004; 103: 1815–1822.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Ichikawa N, Iwabuchi K, Kurihara H, Ishii K, Kobayashi T, Sasaki T et al. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci 2009; 122: 289–299.
Tabe Y, Sebasigari D, Jin L, Rudelius M, Davies-Hill T, Miyake K et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009; 15: 933–942.
Dillmann F, Veldwijk MR, Laufs S, Sperandio M, Calandra G, Wenz F et al. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to imatinib and nilotinib. Leuk Lymphoma 2009; 50: 1676–1686.
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ . SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121.
Hibbs ML, Dunn AR . Lyn, a src-like tyrosine kinase. Int J Biochem Cell Biol 1997; 29: 397–400.
Giri B, Dixit VD, Ghosh MC, Collins GD, Khan IU, Madara K et al. CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur J Immunol 2007; 37: 2104–2116.
Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML . CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res 2008; 6: 446–457.
Dermine JF, Duclos S, Garin J, St-Louis F, Rea S, Parton RG et al. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem 2001; 276: 18507–18512.
Young RM, Holowka D, Baird B . A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem 2003; 278: 20746–20752.
Cebo C, Da Rocha S, Wittnebel S, Turhan AG, Abdelali J, Caillat-Zucman S et al. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J Immunol 2006; 176: 864–872.
Limatola C, Massa V, Lauro C, Catalano M, Giovanetti A, Nuccitelli S et al. Evidence for a role of glycosphingolipids in CXCR4-dependent cell migration. FEBS Lett 2007; 581: 2641–2646.
Sorice M, Garofalo T, Misasi R, Longo A, Mattei V, Sale P et al. Evidence for cell surface association between CXCR4 and ganglioside GM3 after gp120 binding in SupT1 lymphoblastoid cells. FEBS Lett 2001; 506: 55–60.
Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL . Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841–848.
Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood 2009; 113: 6215–6224.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009; 113: 6206–6214.
Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009; 113: 4341–4351.
Ghittoni R, Napolitani G, Benati D, Ulivieri C, Patrussi L, Laghi Pasini F et al. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur J Immunol 2006; 36: 2885–2893.
Yang YC, Huang WF, Chuan LM, Xiao DW, Zeng YL, Zhou DA et al. In vitro and in vivo study of cell growth inhibition of simvastatin on chronic myelogenous leukemia cells. Chemotherapy 2008; 54: 438–446.
Müller-Tidow C, Kiehl M, Sindermann JR, Probst M, Banger N, Zühlsdorf M et al. Synergistic growth inhibitory effects of interferon-alpha and lovastatin on bcr-abl positive leukemic cells. Int J Oncol 2003; 23: 151–158.
Kornblau SM, Banker DE, Stirewalt D, Shen D, Lemker E, Verstovsek E et al. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose Ara-C: a phase 1 study. Blood 2007; 109: 2999–3006.
Sison E, McIntyre E, Magoon D, Brown P . Upregulation of surface CXCR4 in response to chemotherapy confers a stromal-mediated survival advantage in acute leukemia. Blood 2010; 116, : Abstract 2734, 1127.
Acknowledgements
We thank Drs Eri Hirasawa, Zhihong Zeng and Akimasa Someya for their invaluable help and discussion, and Tomomi Ikeda, Takako Shigihara-Ikegami, Akemi Koyanagi and Hiroaki Miyajima for their technical assistance. We thank Kathryn Hale for manuscript review. This work was supported by a Grant-in-Aid for Scientific Research of the Japan Science and Technology Agency, the Japan Leukemia Research Fund, the Osaka Cancer Research Fund and the Project Research Program from Juntendo University School of Medicine (to YT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Tabe, Y., Jin, L., Iwabuchi, K. et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia 26, 883–892 (2012). https://doi.org/10.1038/leu.2011.291
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.291
Keywords
This article is cited by
-
Stem cell persistence in CML is mediated by extrinsically activated JAK1-STAT3 signaling
Leukemia (2019)
-
Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells
Journal of Hematology & Oncology (2018)
-
Membrane perturbation through novel cell-penetrating peptides influences intracellular accumulation of imatinib mesylate in CML cells
Cell Biology and Toxicology (2018)
-
Mtss1 is a critical epigenetically regulated tumor suppressor in CML
Leukemia (2016)
-
Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking
Leukemia (2015)