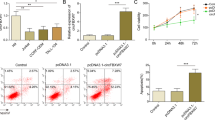
Abstract
MicroRNAs (miRNAs) are a family of 19–24 nucleotide non-coding RNAs with posttranscriptional regulatory functions. The involvement of miRNAs in normal hematopoiesis implies that deregulated miRNAs might contribute to leukemogenesis. To date, although certain miRNAs have been established a clear oncogenic role in hematological malignancies, other individual miRNAs potentially involved in human leukemogenesis still remain elusive. In this report, we showed that miR-142-3p was upregulated in human T-leukemic cell lines and primary T-leukemic cells isolated from T-cell acute lymphoblastic leukemia (T-ALL) patients and its expressive levels were correlated with patients’ prognosis. Such an oncogenic role of miR-142-3p could be explained by its targeting cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) and glucocorticoid receptor alpha (GRα). High levels of miR-142-3p resulted in low levels of cAMP and weak activity of PKA, thus relieving the inhibitory effect of PKA on T-leukemic cell proliferation. Meanwhile, miR-142-3p decreased GRα protein expression by directly targeting the 3′-untranslational region of GRα mRNA, leading to glucocorticoid resistance. Transfection of the miR-142-3p inhibitor effectively converted glucocorticoid resistance, because of the resultant increase of GRα expression and PKA activity. These findings suggest that miR-142-3p is critical in T-cell leukemogenesis and may serve as a potential therapeutic target in T-ALL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Iorio MV, Croce CM . MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 2009; 27: 5848–5856.
Lee YS, Dutta A . MicroRNAs in cancer. Annu Rev Pathol 2009; 4: 199–227.
Esquela-Kerscher A, Slack FJ . Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23: 4051–4060.
Cai X, Hagedorn CH, Cullen BR . Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004; 10: 1957–1966.
Ambros V . The functions of animal microRNAs. Nature 2004; 431: 350–355.
Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 2007; 67: 2456–2468.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D et al. MicroRNA expression profiles classify human cancers. Nature 2005; 435: 834–838.
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T . Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 2007; 39: 673–677.
Zhang B, Pan X, Cobb GP, Anderson TA . microRNAs as oncogenes and tumor suppressors. Dev Biol 2007; 302: 1–12.
Yendamuri S, Calin GA . The role of microRNA in human leukemia: a review. Leukemia 2009; 23: 1257–1263.
Garzon R, Croce CM . MicroRNAs in normal and malignant hematopoiesis. Curr Opin Hematol 2008; 15: 352–358.
Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA 2005; 102: 3627–3632.
Wang M, Tan LP, Dijkstra MK, van Lom K, Robertus JL, Harms G et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J Pathol 2008; 215: 13–20.
Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU et al. Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood 2007; 109: 4399–4405.
Landais S, Landry S, Legault P, Rassart E . Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res 2007; 67: 5699–5707.
Chen CZ, Li L, Lodish HF, Bartel DP . MicroRNAs modulate hematopoietic lineage differentiation. Science 2004; 303: 83–86.
Merkerova M, Belickova M, Bruchova H . Differential expression of microRNAs in hematopoietic cell lineages. Eur J Haematol 2008; 81: 304–310.
Bellon M, Lepelletier Y, Hermine O, Nicot C . Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 2009; 113: 4914–4917.
Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX et al. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep 2009; 10: 180–185.
Tamir A, Granot Y, Isakov N . Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. J Immunol 1996; 157: 1514–1522.
Lerner A, Kim DH, Lee R . The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma 2000; 37: 39–51.
Gauwerky CE, Huebner K, Isobe M, Nowell PC, Croce CM . Activation of MYC in a masked t(8;17) translocation results in an aggressive B-cell leukemia. Proc Natl Acad Sci USA 1989; 86: 8867–8871.
Skalhegg BS, Tasken K . Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 2000; 5: D678–D693.
Schwede F, Maronde E, Genieser H, Jastorff B . Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther 2000; 87: 199–226.
Rothermel JD, Stec WJ, Baraniak J, Jastorff B, Botelho LH . Inhibition of glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3′,5′-phosphorothioate. J Biol Chem 1983; 258: 12125–12128.
Frankfurt O, Rosen ST . Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol 2004; 16: 553–563.
Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E . Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res 2008; 101: 127–248.
Ploner C, Schmidt S, Presul E, Renner K, Schröcksnadel K, Rainer J et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance in acute lymphoblastic leukemia. J Steroid Biochem Mol Biol 2005; 93: 153–160.
Schmidt S, Irving JA, Minto L, Matheson E, Nicholson L, Ploner A et al. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB J 2006; 20: 2600–2602.
Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R . Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ 2004; 11: S45–S55.
Medh RD, Saeed MF, Johnson BH, Thompson EB . Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression. Cancer Res 1998; 58: 3684–3693.
Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X . Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. J Biol Chem 2007; 282: 37370–37377.
Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA et al. miRNA profiling of naïve, effector and memory CD8 T cells. PLoS One 2007; 2: e1020.
Pui CH, Relling MV, Downing JR . Acute lymphoblastic leukemia. N Engl J Med 2004; 350: 1535–1548.
Yudt MR, Cidlowski JA . The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol 2002; 16: 1719–1726.
Hauk PJ, Goleva E, Strickland I, Vottero A, Chrousos GP, Kisich KO et al. Increased glucocorticoid receptor Beta expression converts mouse hybridoma cells to a corticosteroid-insensitive phenotype. Am J Respir Cell Mol Biol 2002; 27: 361–367.
Irving JA, Minto L, Bailey S, Hall AG . Loss of heterozygosity and somatic mutations of the glucocorticoid receptor gene are rarely found at relapse in pediatric acute lymphoblastic leukemia but may occur in a subpopulation early in the disease course. Cancer Res 2005; 65: 9712–9718.
Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML et al. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood 2006; 108: 1045–1049.
Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R . Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ 2004; 11: S65–S72.
Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW et al. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 1999; 93: 2360–2368.
Taganov KD, Boldin MP, Chang KJ, Baltimore D . NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103: 12481–12486.
Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M . Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-kappaB pathway. Nucleic Acids Res 2008; 36: 6608–6619.
McKay LI, Cidlowski JA . Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol 1998; 12: 45–56.
Tao Y, Williams-Skipp C, Scheinman RI . Mapping of glucocorticoid receptor DNA binding domain surfaces contributing to transrepression of NF-kappa B and induction of apoptosis. J Biol Chem 2001; 276: 2329–2332.
Ramstad C, Sundvold V, Johansen HK, Lea T . cAMP-dependent protein kinase (PKA) inhibits T cell activation by phosphorylating ser-43 of raf-1 in the MAPK/ERK pathway. Cell Signal 2000; 12: 557–563.
van Oirschot BA, Stahl M, Lens SM, Medema RH . Protein kinase A regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem 2001; 276: 33854–33860.
Bodor J, Feigenbaum L, Bodorova J, Bare C, Reitz Jr MS, Gress RE . Suppression of T-cell responsiveness by inducible cAMP early repressor (ICER). J Leukoc Biol 2001; 69: 1053–1059.
Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T . Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol 2005; 35: 31–41.
Acknowledgements
We thank the patients for their cooperation and confidence. This study was supported by the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (30911120482), the National Science Foundation of China (30770911, 81070449), the Program for New Century Excellent Talents in University (NCET-08-0219) and the Beijing Natural Science Foundation (7112139).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Lv, M., Zhang, X., Jia, H. et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-α and cAMP/PKA pathways. Leukemia 26, 769–777 (2012). https://doi.org/10.1038/leu.2011.273
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.273
Keywords
This article is cited by
-
Non-coding RNAs in leukemia drug resistance: new perspectives on molecular mechanisms and signaling pathways
Annals of Hematology (2023)
-
Chronic myeloid leukemia stem cells: targeting therapeutic implications
Stem Cell Research & Therapy (2021)
-
The Role of Epigenetics in the Chronic Sinusitis with Nasal Polyp
Current Allergy and Asthma Reports (2021)
-
The role of CDC25C in cell cycle regulation and clinical cancer therapy: a systematic review
Cancer Cell International (2020)
-
A unique insight into the MiRNA profile during genital chlamydial infection
BMC Genomics (2019)