Abstract
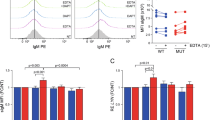
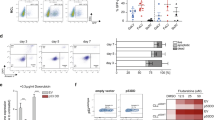
A major pathogenetic mechanism in classical Hodgkin lymphoma (cHL) is constitutive activation of canonical nuclear factor-κB (NF-κB) p50/p65 signaling, controlling lymphoma cell proliferation and survival. Recently, we demonstrated that aberrant Notch1 activity is a negative regulator of the B cell program in B cell-derived Hodgkin and Reed–Sternberg (HRS) cells. Despite abundant evidence for a complex context-dependent cross talk between Notch and NF-κB signaling in hematopoietic cells, it is unknown whether these pathways interact in HRS cells. Here, we show that Notch-signaling inhibition in HRS cells by the γ-secretase inhibitor (GSI) XII results in decreased alternative p52/RelB NF-κB signaling, interfering with processing of the NF-κB2 gene product p100 into its active form p52. As a result, expression of Notch and NF-κB target genes is reduced, and survival of HRS cells is impaired. Stimulation of alternative NF-κB signaling in the Hodgkin cell line L540cy by activation of the CD30 receptor rescued GSI-mediated loss of cell viability and apoptosis induction. Our data reveal that Notch is an essential upstream regulator of alternative NF-κB signaling and indicate cross talk between both the pathways in HRS cells. Therefore, we suggest that targeting the Notch pathway is a promising therapeutic option in cHL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kopan R, Ilagan MX . The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137: 216–233.
Radtke F, Fasnacht N, Macdonald HR . Notch signaling in the immune system. Immunity 2010; 32: 14–27.
Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H, Dorken B . Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood 2002; 99: 3398–3403.
Kochert K, Ullrich K, Kreher S, Aster JC, Kitagawa M, Johrens K et al. High-level expression of Mastermind-like 2 contributes to aberrant activation of the NOTCH signaling pathway in human lymphomas. Oncogene 2011; 30: 1831–1840.
Jundt F, Acikgoz O, Kwon SH, Schwarzer R, Anagnostopoulos I, Wiesner B et al. Aberrant expression of Notch1 interferes with the B-lymphoid phenotype of neoplastic B cells in classical Hodgkin lymphoma. Leukemia 2008; 22: 1587–1594.
Osipo C, Golde TE, Osborne BA, Miele LA . Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest 2008; 88: 11–17.
Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K et al. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed–Sternberg cells. Blood 1996; 87: 4340–4347.
Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest 1997; 100: 2961–2969.
Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C et al. Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosis. Blood 2003; 102: 1028–1034.
Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed–Sternberg cells. Blood 1999; 94: 3129–3134.
Emmerich F, Theurich S, Hummel M, Haeffker A, Vry MS, Dohner K et al. Inactivating I kappa B epsilon mutations in Hodgkin/Reed–Sternberg cells. J Pathol 2003; 201: 413–420.
Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dorken B, Scheidereit C . Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/Reed–Sternberg cells. Oncogene 1999; 18: 943–953.
Horie R, Watanabe T, Morishita Y, Ito K, Ishida T, Kanegae Y et al. Ligand-independent signaling by overexpressed CD30 drives NF-kappaB activation in Hodgkin–Reed—Sternberg cells. Oncogene 2002; 21: 2493–2503.
Kuppers R . The biology of Hodgkin's lymphoma. Nat Rev Cancer 2009; 9: 15–27.
Nonaka M, Horie R, Itoh K, Watanabe T, Yamamoto N, Yamaoka S . Aberrant NF-kappaB2/p52 expression in Hodgkin/Reed–Sternberg cells and CD30-transformed rat fibroblasts. Oncogene 2005; 24: 3976–3986.
Saitoh Y, Yamamoto N, Dewan MZ, Sugimoto H, Martinez Bruyn VJ, Iwasaki Y et al. Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed–Sternberg cells. Blood 2008; 111: 5118–5129.
Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S et al. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med 2002; 196: 605–617.
Guo F, Sun A, Wang W, He J, Hou J, Zhou P et al. TRAF1 is involved in the classical NF-kappaB activation and CD30-induced alternative activity in Hodgkin's lymphoma cells. Mol Immunol 2009; 46: 2441–2448.
Wright CW, Rumble JM, Duckett C . CD30 activates both the canonical and alternative NF-kB pathways in anaplastic large cell lymphoma cells. J Biol Chem 2007; 282: 10252–10262.
Hirsch B, Hummel M, Bentink S, Fouladi F, Spang R, Zollinger R et al. CD30-induced signaling is absent in Hodgkin's cells but present in anaplastic large cell lymphoma cells. Am J Pathol 2008; 172: 510–520.
Bergmann M, Hart L, Lindsay M, Barnes PJ, Newton R . Ikappa B alpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription. Requirement for an additional activation pathway. J Biol Chem 1998; 273: 6607–6610.
Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI . Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood 2008; 111: 2220–2229.
Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol 2006; 7: 207–215.
Scheidereit C . IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 2006; 25: 6685–6705.
Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A et al. Expression of CCR5 receptors on Reed–Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer 2008; 122: 769–776.
Aldinucci D, Poletto D, Gloghini A, Nanni P, Degan M, Perin T et al. Expression of functional interleukin-3 receptors on Hodgkin and Reed–Sternberg cells. Am J Pathol 2002; 160: 585–596.
Kapp U, Yeh WC, Patterson B, Elia AJ, Kagi D, Ho A et al. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed–Sternberg cells. J Exp Med 1999; 189: 1939–1946.
Schwarzer R, Kaiser M, Acikgoez O, Heider U, Mathas S, Preissner R et al. Notch inhibition blocks multiple myeloma cell-induced osteoclast activation. Leukemia 2008; 22: 2273–2277.
Rosati E, Sabatini R, Rampino G, Tabilio A, Di Ianni M, Fettucciari K et al. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood 2009; 113: 856–865.
Fiumara P, Snell V, Li Y, Mukhopadhyay A, Younes M, Gillenwater AM et al. Functional expression of receptor activator of nuclear factor kappaB in Hodgkin disease cell lines. Blood 2001; 98: 2784–2790.
Oswald F, Liptay S, Adler G, Schmid RM . NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol 1998; 18: 2077–2088.
Alison MR, Lim SM, Nicholson LJ . Cancer stem cells: problems for therapy? J Pathol 2011; 223: 147–161.
Deangelo DJSR, Silverman LB, Stock W, Attar EC, Fearen I et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J Clin Oncol 2006; 24: 357s.
Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med 2009; 15: 50–58.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, TRR54, TPB6 to FJ and BD) and the Wilhelm-Sander Stiftung (2006.069.2). We thank Katharina Pardon and Alexander Haake for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Schwarzer, R., Dörken, B. & Jundt, F. Notch is an essential upstream regulator of NF-κB and is relevant for survival of Hodgkin and Reed–Sternberg cells. Leukemia 26, 806–813 (2012). https://doi.org/10.1038/leu.2011.265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.265
Keywords
This article is cited by
-
Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia-reperfusion injury through crosstalk with the NF-κB pathway
Journal of Neuroinflammation (2019)
-
The dynamic nature of senescence in cancer
Nature Cell Biology (2019)
-
Comprehensive bioinformation analysis of the miRNA of PLCE1 knockdown in esophageal squamous cell carcinoma
Molecular and Cellular Biochemistry (2018)
-
The persistent dynamic secrets of senescence
Nature Cell Biology (2016)
-
Scutellarin as a Potential Therapeutic Agent for Microglia-Mediated Neuroinflammation in Cerebral Ischemia
NeuroMolecular Medicine (2016)