Abstract
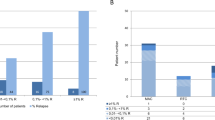
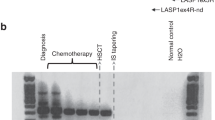
Relapse of malignant disease remains the major complication in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) after hematopoietic cell transplantation (HCT) with reduced-intensity conditioning (RIC). In this study, we investigated the predictive value of disease-specific markers (DSMs), donor chimerism (DC) analysis of unsorted (UDC) or CD34+ sorted cells and Wilms’ tumor gene 1 (WT1) expression. Eighty-eight patients with AML or MDS were monitored after allogenic HCT following 2 Gy total-body irradiation with (n=84) or without (n=4) fludarabine 3 × 30 mg/m2, followed by cyclosporin A and mycophenolate mofetil. DSMs were determined by fluorescence in situ hybridization (FISH) and WT1 expression by real-time polymerase chain reaction. Chimerism analysis was performed on unsorted or CD34+ sorted cells, by FISH or short tandem repeat polymerase chain reaction. Twenty-one (24%) patients relapsed within 4 months after HCT. UDC, CD34+ DC and WT1 expression were each significant predictors of relapse with sensitivities ranging from 53 to 79% and specificities of 82–91%. Relapse within 28 days was excluded almost entirely on the basis of WT1 expression combined with CD34+ DC kinetics. Monitoring of WT1 expression and CD34+ DC predict relapse of AML and MDS after RIC-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Storb R . Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Pract Res Clin Haematol 2007; 20: 85–90.
Niederwieser D, Lange T, Cross M, Basara N, Al-Ali H . Reduced intensity conditioning (RIC) haematopoietic cell transplants in elderly patients with AML. Best Pract Res Clin Haematol 2006; 19: 825–838.
Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol 2005; 23: 9387–9393.
Hallemeier CL, Girgis MD, Blum WG, Brown RA, Khoury HJ, Devine SM et al. Long-term remissions in patients with myelodysplastic syndrome and secondary acute myelogenous leukemia undergoing allogeneic transplantation following a reduced intensity conditioning regimen of 550 cGy total body irradiation and cyclophosphamide. Biol Blood Marrow Transplant 2006; 12: 749–757.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol 2006; 24: 444–453.
Al-Ali HK, Nehring C, Krahl R, Becker C, Leiblein S, Edelmann J et al. Donor CD34+ cell chimerism at day 28 and chronic graft-versus-host disease (GvHD) but not high-risk cytogenetics influence outcome of allogeneic hematopoetic cell transplantation (HCT) following reduced intensity conditioning (RIC) in patients with AML and MDS. ASH Annual Meeting Abstracts 2006; 108: 547.
Mielcarek M, Martin PJ, Maloney DG, Storb R, Sandmaier BM . Outcomes among patients with recurrent high-risk hematologic malignancy after nonmyeloablative versus myeloablative allogeneic hematopoietic cell transplantation. ASH Annual Meeting Abstracts 2006; 108: 262.
Lange T, Deininger M, Brand R, Hegenbart U, Al-Ali H, Krahl R et al. BCR-ABL transcripts are early predictors for hematological relapse in chronic myeloid leukemia after hematopoietic cell transplantation with reduced intensity conditioning. Leukemia 2004; 18: 1468–1475.
Kern W, Schoch C, Haferlach T, Schnittger S . Monitoring of minimal residual disease in acute myeloid leukemia. Crit Rev Oncol Hematol 2005; 56: 283–309.
Gozzetti A, Le Beau MM . Fluorescence in situ hybridization: uses and limitations. Semin Hematol 2000; 37: 320–333.
Thiede C . Diagnostic chimerism analysis after allogeneic stem cell transplantation: new methods and markers. Am J Pharmacogenom 2004; 4: 177–187.
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746.
Gorello P, Cazzaniga G, Alberti F, Dell’Oro MG, Gottardi E, Specchia G et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006; 20: 1103–1108.
Schnittger S, Schoch C, Kern W, Hiddemann W, Haferlach T . FLT3 length mutations as marker for follow-up studies in acute myeloid leukaemia. Acta Haematol 2004; 112: 68–78.
Scholl S, Krause C, Loncarevic IF, Muller R, Kunert C, Wedding U et al. Specific detection of Flt3 point mutations by highly sensitive real-time polymerase chain reaction in acute myeloid leukemia. J Lab Clin Med 2005; 145: 295–304.
Cilloni D, Saglio G . WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Haematol 2004; 112: 79–84.
Keilholz U, Menssen HD, Gaiger A, Menke A, Oji Y, Oka Y et al. Wilms’ tumour gene 1 (WT1) in human neoplasia. Leukemia 2005; 19: 1318–1323.
Ostergaard M, Olesen LH, Hasle H, Kjeldsen E, Hokland P . WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients—results from a single-centre study. Br J Haematol 2004; 125: 590–600.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol 2009; 27: 5195–5201.
Weisser M, Kern W, Rauhut S, Schoch C, Hiddemann W, Haferlach T et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia 2005; 19: 1416–1423.
Lapillonne H, Renneville A, Auvrignon A, Flamant C, Blaise A, Perot C et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol 2006; 24: 1507–1515.
Cilloni D, Messa F, Arruga F, Defilippi I, Gottardi E, Fava M et al. Early prediction of treatment outcome in acute myeloid leukemia by measurement of WT1 transcript levels in peripheral blood samples collected after chemotherapy. Haematologica 2008; 93: 921–924.
Osborne D, Frost L, Tobal K, Liu Yin JA . Elevated levels of WT1 transcripts in bone marrow harvests are associated with a high relapse risk in patients autografted for acute myeloid leukaemia. Bone Marrow Transplant 2005; 36: 67–70.
Ogawa H, Ikegame K, Kawakami M, Tamaki H . WT1 gene transcript assay for relapse in acute leukemia after transplantation. Leuk Lymphoma 2004; 45: 1747–1753.
Ogawa H, Tamaki H, Ikegame K, Soma T, Kawakami M, Tsuboi A et al. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood 2003; 101: 1698–1704.
Candoni A, Tiribelli M, Toffoletti E, Cilloni D, Chiarvesio A, Michelutti A et al. Quantitative assessment of WT1 gene expression after allogeneic stem cell transplantation is a useful tool for monitoring minimal residual disease in acute myeloid leukemia. Eur J Haematol 2009; 82: 61–68.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood 2003; 101: 1620–1629.
Bryant E, Martin PJ . Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Transplantation, 2 edn. Boston, 1999, pp 197–206.
Bumm T, Muller C, Al-Ali HK, Krohn K, Shepherd P, Schmidt E et al. Emergence of clonal cytogenetic abnormalities in Ph− cells in some CML patients in cytogenetic remission to imatinib but restoration of polyclonal hematopoiesis in the majority. Blood 2003; 101: 1941–1949.
Lange T, Niederwieser DW, Deininger MW . Residual disease in chronic myeloid leukemia after induction of molecular remission. N Engl J Med 2003; 349: 1483–1484.
Ihaka R, Gentleman R . R a language for data analysis and graphics. J Comp Graph Stat 1996; 5: 299–314.
Bornhauser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 2009; 94: 1613–1617.
Bacher U, Kern W, Schoch C, Schnittger S, Hiddemann W, Haferlach T . Evaluation of complete disease remission in acute myeloid leukemia: a prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer 2006; 106: 839–847.
Mancini M, Cedrone M, Diverio D, Emanuel B, Stul M, Vranckx H et al. Use of dual-color interphase FISH for the detection of inv(16) in acute myeloid leukemia at diagnosis, relapse and during follow-up: a study of 23 patients. Leukemia 2000; 14: 364–368.
Zeiser R, Spyridonidis A, Wasch R, Ihorst G, Grullich C, Bertz H et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia 2005; 19: 814–821.
Nyvold CG, Stentoft J, Braendstrup K, Melsvik D, Moestrup SK, Juhl-Christensen C et al. Wilms’ tumor 1 mutation accumulated during therapy in acute myeloid leukemia: Biological and clinical implications. Leukemia 2006; 20: 2051–2054.
Gaidzik VI, Schlenk RF, Moschny S, Becker A, Bullinger L, Corbacioglu A et al. Prognostic impact of WT1 mutations in cytogenetically normal acute myeloid leukemia (AML): A study of the German–Austrian AML Study Group (AMLSG). Blood 2009; 113: 4505–4511.
Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107: 1116–1123.
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 2859–2867.
Bethge WA, Storer BE, Maris MB, Flowers ME, Maloney DG, Chauncey TR et al. Relapse or progression after hematopoietic cell transplantation using nonmyeloablative conditioning: effect of interventions on outcome. Exp Hematol 2003; 31: 974–980.
Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 2009; 113: 6567–6571.
Cross M, Jaekel N, Krahl R, Junghanss C, Maschmeyer G, Tran T et al. Low levels of global (LINE) and CDH13 methylation at diagnosis and rapid clearance of marrow blasts correlate with a better haematological response to azacitidine in patients with newly diagnosed and refractory/relapsed AML not eligible for or resistant to chemotherapy: a multi-centre phase I/II-study of the East German Haematology and Oncology Study Group (OSHO). ASH Annual Meeting Abstracts 2009; 114: 2642.
Acknowledgements
We thank Janet Bogardt, Dagmar Crohn, Christa Döring, Christina Franke, Christine Günther, Evelyn Hennig, Ines Kovacs, Rainer Krahl, Christel Müller and Scarlet Musiol for their excellent assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, T., Hubmann, M., Burkhardt, R. et al. Monitoring of WT1 expression in PB and CD34+ donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 25, 498–505 (2011). https://doi.org/10.1038/leu.2010.283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.283
Keywords
This article is cited by
-
A high hematopoietic cell transplantation comorbidity Index (HCT-CI) does not impair outcomes after non-myeloablative allogeneic stem cell transplantation in acute myeloid leukemia patients 60 years or older
Bone Marrow Transplantation (2023)
-
Prognostic impact of the AML ELN2022 risk classification in patients undergoing allogeneic stem cell transplantation
Blood Cancer Journal (2022)
-
Impact on outcomes of mixed chimerism of bone marrow CD34+ sorted cells after matched or haploidentical allogeneic stem cell transplantation for myeloid malignancies
Bone Marrow Transplantation (2022)
-
Prospective phase II study of preemptive chimerism-driven reduction of immunosuppression after non-myeloablative conditioning—Eudract #: 2007-002420-15
Bone Marrow Transplantation (2022)
-
Dynamic change in peripheral blood WT1 mRNA levels within three cycles of azacitidine predict treatment response in patients with high-risk myelodysplastic syndromes
Annals of Hematology (2022)