Abstract
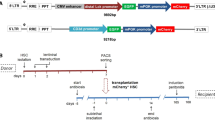
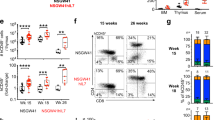
X-linked agammaglobulinemia (XLA) is the most common primary immunodeficiency (PID) in man and caused by mutations in the Bruton’s tyrosine kinase (BTK) gene. XLA is characterized by a B-cell differentiation arrest in bone marrow, absence of mature B cells and immunoglobulins (Igs), and recurrent bacterial infections. We used self-inactivating lentiviral vectors expressing codon-optimized human BTK under the control of three different ubiquitous or B cell-specific promoters. Btk−/− mice engrafted with transduced cells showed correction of both precursor B-cell and peripheral B-cell development. Lentiviral vectors containing the wildtype BTK sequence did not correct the phenotype. All treated mice with codon-optimized BTK exhibited the recovery of B1 cells in the peritoneal cavity, and of serum IgM and IgG3 levels. Calcium mobilization responses upon B-cell receptor stimulation as well as in vivo responses to T cell-independent antigens were restored. Viral promoters overexpressing BTK >100-fold above normal resulted in erythro-myeloid proliferations independent of insertional mutagenesis. However, transplantation into secondary Btk−/− recipients using cellular promoters resulted in functional restoration of peripheral B cells and IgM levels, without any adverse effects. In conclusion, transduction of human BTK corrects B-cell development and antigen-specific antibody responses in Btk−/− mice, thus indicating the feasibility of lentiviral gene therapy for XLA, provided that BTK expression does not vastly exceed normal levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Fischer A . Human primary immunodeficiency diseases: a perspective. Nat Immunol 2004; 5: 23–30.
Cavazzana-Calvo M, Fischer A . Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest 2007; 117: 1456–1465.
Pike-Overzet K, van der Burg M, Wagemaker G, van Dongen JJ, Staal FJ . New insights and unresolved issues regarding insertional mutagenesis in X-linked SCID gene therapy. Mol Ther 2007; 15: 1910–1916.
Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993; 361: 226–233.
Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 1993; 72: 279–290.
Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M . The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays 2001; 23: 436–446.
Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 2004; 104: 1191–1197.
Schmidt U, Boucheron N, Unger B, Ellmeier W . The role of Tec family kinases in myeloid cells. Int Arch Allergy Immunol 2004; 134: 65–78.
Whyburn LR, Halcomb KE, Contreras CM, Lowell CA, Witte ON, Satterthwaite AB . Reduced dosage of Bruton’s tyrosine kinase uncouples B cell hyperresponsiveness from autoimmunity in lyn-/- mice. J Immunol 2003; 171: 1850–1858.
de Weers M, Verschuren MC, Kraakman ME, Mensink RG, Schuurman RK, van Dongen JJ et al. The Bruton’s tyrosine kinase gene is expressed throughout B cell differentiation, from early precursor B cell stages preceding immunoglobulin gene rearrangement up to mature B cell stages. Eur J Immunol 1993; 23: 3109–3114.
de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW . Mutation analysis of the Bruton’s tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet 1994; 3: 161–166.
Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol 1994; 152: 557–565.
von Lindern M, Schmidt U, Beug H . Control of erythropoiesis by erythropoietin and stem cell factor: a novel role for Bruton’s tyrosine kinase. Cell Cycle 2004; 3: 876–879.
Aoki Y, Isselbacher KJ, Cherayil BJ, Pillai S . Tyrosine phosphorylation of Blk and Fyn Src homology 2 domain-binding proteins occurs in response to antigen-receptor ligation in B cells and constitutively in pre-B cells. Proceedings of the National Academy of Sciences of the United States of America 1994; 91: 4204–4208.
Aoki Y, Isselbacher KJ, Pillai S . Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc Natl Acad Sci USA 1994; 91: 10606–10609.
Lindvall JM, Blomberg KE, Valiaho J, Vargas L, Heinonen JE, Berglof A et al. Bruton’s tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev 2005; 203: 200–215.
Noordzij JG, de Bruin-Versteeg S, Hartwig NG, Weemaes CM, Gerritsen EJ, Bernatowska E et al. XLA patients with BTK splice-site mutations produce low levels of wild-type BTK transcripts. J Clin Immunol 2002; 22: 306–318.
van Zelm MC, Geertsema C, Nieuwenhuis N, de Ridder D, Conley ME, Schiff C et al. Gross deletions involving IGHM, BTK, or Artemis: a model for genomic lesions mediated by transposable elements. Am J Hum Genet 2008; 82: 320–332.
Noordzij JG, de Bruin-Versteeg S, Comans-Bitter WM, Hartwig NG, Hendriks RW, de Groot R et al. Composition of precursor B-cell compartment in bone marrow from patients with X-linked agammaglobulinemia compared with healthy children. Pediatr Res 2002; 51: 159–168.
Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G et al. Defective B cell development and function in Btk-deficient mice. Immunity 1995; 3: 283–299.
Wicker LS, Scher I . X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol 1986; 124: 87–101.
Witkowski J, Forrester LM, Ansell JD, Micklem HS . Influence of the xid mutation on B lymphocyte development in adult mice. Adv Exp Med Biol 1985; 186: 47–55.
Middendorp S, Dingjan GM, Hendriks RW . Impaired precursor B cell differentiation in Bruton’s tyrosine kinase-deficient mice. J Immunol 2002; 168: 2695–2703.
Conley ME, Broides A, Hernandez-Trujillo V, Howard V, Kanegane H, Miyawaki T et al. Genetic analysis of patients with defects in early B-cell development. Immunol rev 2005; 203: 216–234.
Yu PW, Tabuchi RS, Kato RM, Astrakhan A, Humblet-Baron S, Kipp K et al. Sustained correction of B-cell development and function in a murine model of X-linked agammaglobulinemia (XLA) using retroviral-mediated gene transfer. Blood 2004; 104: 1281–1290.
Ellmeier W, Jung S, Sunshine MJ, Hatam F, Xu Y, Baltimore D et al. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. J Exp Med 2000; 192: 1611–1624.
Moreau T, Barlogis V, Bardin F, Nunes JA, Calmels B, Chabannon C et al. Development of an enhanced B-specific lentiviral vector expressing BTK: a tool for gene therapy of XLA. Gene Ther 2008; 15: 942–952.
Moreau T, Calmels B, Barlogis V, Michel G, Tonnelle C, Chabannon C . Potential application of gene therapy to X-linked agammaglobulinemia. Curr Gene Ther 2007; 7: 284–294.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet 2004; 364: 2181–2187.
Thornhill SI, Schambach A, Howe SJ, Ulaganathan M, Grassman E, Williams D et al. Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol Ther 2008; 16: 590–598.
Deichmann A, Hacein-Bey-Abina S, Schmidt M, Garrigue A, Brugman MH, Hu J et al. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J Clin Invest 2007; 117: 2225–2232.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006; 24: 687–696.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F . HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002; 110: 521–529.
Shou Y, Ma Z, Lu T, Sorrentino BP . Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc Natl Acad Sci USA 2006; 103: 11730–11735.
Modlich U, Bohne J, Schmidt M, von Kalle C, Knoss S, Schambach A et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 2006; 108: 2545–2553.
Modlich U, Schambach A, Brugman MH, Wicke DC, Knoess S, Li Z et al. Leukemia induction after a single retroviral vector insertion in Evi1 or Prdm16. Leukemia 2008; 22: 1519–1528.
Hendriks RW, de Bruijn MF, Maas A, Dingjan GM, Karis A, Grosveld F . Inactivation of Btk by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. Embo J 1996; 15: 4862–4872.
Schambach A, Galla M, Maetzig T, Loew R, Baum C . Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol Ther 2007; 15: 1167–1173.
Werner M, Kraunus J, Baum C, Brocker T . B-cell-specific transgene expression using a self-inactivating retroviral vector with human CD19 promoter and viral post-transcriptional regulatory element. Gene Ther 2004; 11: 992–1000.
Baum C, Itoh K, Meyer J, Laker C, Ito Y, Ostertag W . The potent enhancer activity of the polycythemic strain of spleen focus-forming virus in hematopoietic cells is governed by a binding site for Sp1 in the upstream control region and by a unique enhancer core motif, creating an exclusive target for PEBP/CBF. J virol 1997; 71: 6323–6331.
Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol Ther 2006; 13: 391–400.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 2009; 113: 546–554.
van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med 2006; 354: 1901–1912.
Staal FJ, Luis TC, Tiemessen MM . WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 2008; 8: 581–593.
Modlich U, Kustikova OS, Schmidt M, Rudolph C, Meyer J, Li Z et al. Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood 2005; 105: 4235–4246.
Rohrer J, Conley ME . Correction of X-linked immunodeficient mice by competitive reconstitution with limiting numbers of normal bone marrow cells. Blood 1999; 94: 3358–3365.
Moreno-Carranza B, Gentsch M, Stein S, Schambach A, Santilli G, Rudolf E et al. Transgene optimization significantly improves SIN vector titers, gp91phox expression and reconstitution of superoxide production in X-CGD cells. Gene Ther 2009; 16: 111–118.
Tanabe H, Miyake K, Shimada T . HIV-mediated expression of Btk in hematopoietic stem cells is not sufficient to restore B cell function in X-linked immunodeficient mice. J Nippon Med Sch 2005; 72: 203–212.
Sochorova K, Horvath R, Rozkova D, Litzman J, Bartunkova J, Sediva A et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood 2007; 109: 2553–2556.
Kersseboom R, Middendorp S, Dingjan GM, Dahlenborg K, Reth M, Jumaa H et al. Bruton’s tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in pre-B cells. J Exp Med 2003; 198: 91–98.
Maas A, Dingjan GM, Savelkoul HF, Kinnon C, Grosveld F, Hendriks RW . The X-linked immunodeficiency defect in the mouse is corrected by expression of human Bruton’s tyrosine kinase from a yeast artificial chromosome transgene. Eur J Immunol 1997; 27: 2180–2187.
Staal FJ, Pike-Overzet K, Ng YY, van Dongen JJ . Sola dosis facit venenum. Leukemia in gene therapy trials: a question of vectors, inserts and dosage? Leukemia 2008; 22: 1849–1852.
Cavazzana-Calvo M, Andre-Schmutz I, Dal Cortivo L, Neven B, Hacein-Bey-Abina S, Fischer A . Immune reconstitution after haematopoietic stem cell transplantation: obstacles and anticipated progress. Curr Opin Immunol 2009; 21: 544–548.
Fischer A, Cavazzana-Calvo M . Gene therapy of inherited diseases. Lancet 2008; 371: 2044–2047.
Kerns HM, Ryu BY, Stirling BV, Sather BD, Astrakhan A, Humblet-Baron S, Liggitt D, Rawlings DJ . B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia Blood, e-pub ahead of print 21 January 2010.
Acknowledgements
We would like to thank Tiago Luis, Brigitta Naber, Edwin de Hass and Machteld Tiemessen (Staal laboratory) for their technical assistance and Yvette Caljouw for her assistance at the EDC. This work was supported in part by a Grant from the Translational Gene Therapy Research Program of ZonMw—the Netherlands Organization for Health Research and Development (project no. 43100016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Ng, Y., Baert, M., Pike-Overzet, K. et al. Correction of B-cell development in Btk-deficient mice using lentiviral vectors with codon-optimized human BTK. Leukemia 24, 1617–1630 (2010). https://doi.org/10.1038/leu.2010.140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.140
Keywords
This article is cited by
-
A novel Bruton’s tyrosine kinase inhibitor CC-292 in combination with the proteasome inhibitor carfilzomib impacts the bone microenvironment in a multiple myeloma model with resultant antimyeloma activity
Leukemia (2014)
-
Gene transfer into hematopoietic stem cells as treatment for primary immunodeficiency diseases
International Journal of Hematology (2014)
-
Induced pluripotent stem cells and severe combined immunodeficiency: merely disease modeling or potentially a novel cure?
Pediatric Research (2012)
-
The β-Globin Locus Control Region in Combination With the EF1α Short Promoter Allows Enhanced Lentiviral Vector-mediated Erythroid Gene Expression With Conserved Multilineage Activity
Molecular Therapy (2012)
-
New frontiers of primary antibody deficiencies
Cellular and Molecular Life Sciences (2012)