Abstract
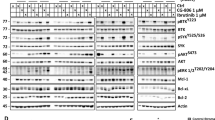
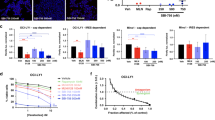
Mantle cell lymphoma (MCL) is a type of aggressive B-cell non-Hodgkin's lymphoma characterized by frequent resistance to conventional chemotherapy. In this study we provided evidence that fenofibrate, which is widely known as an agonist for peroxisome proliferator-activated receptor-α (PPARα), can induce effective apoptosis in treating MCL cells. Addition of fenofibrate to MCL cell lines significantly decreased the number of viable cells by 50% at approximately 20 μM at 72 h. This decrease in cell growth was due to apoptosis, as evidenced by the cleavage of caspase 3 and poly(ADP-ribose) polymerase. The fenofibrate-mediated effects were not significantly affected by GW6471, a specific PPARα antagonist. Using an apoptosis pathway-specific oligonucleotide array, we found that fenofibrate significantly downregulated several pro-survival genes, including tumor necrosis factor-α (TNFα). Importantly, addition of recombinant TNF-α conferred partial protection against fenofibrate-induced apoptosis. Fenofibrate also decreased the nuclear translocation of nuclear factor (NF)-κB-p65 and significantly inhibited the DNA binding of NF-κB in a dose-dependent manner. To conclude, fenofibrate shows efficacy against MCL, and the mechanism can be attributed to its inhibitory effects on the TNF-α/NF-κB signaling axis. In view of the documented safety of fenofibrate in humans, it may provide a valuable therapeutic option for MCL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Desvergne B, Wahli W . Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20: 649–688.
Kersten S, Desvergne B, Wahli W . Roles of PPARs in health and disease. Nature 2000; 405: 421–424.
Evans RM . The steroid and thyroid hormone receptor superfamily. Science 1988; 240: 889–895.
Green S, Wahli W . Peroxisome proliferator-activated receptors: finding the orphan a home. Mol Cell Endocrinol 1994; 100: 149–153.
Chinetti G, Fruchart JC, Staels B . Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 2000; 49: 497–505.
Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998; 393: 790–793.
Delerive P, Fruchart JC, Staels B . Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 2001; 169: 453–459.
Poynter ME, Daynes RA . Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 1998; 273: 32833–32841.
Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 1999; 274: 32048–32054.
Madej A, Okopien B, Kowalski J, Zielinski M, Wysocki J, Szygula B et al. Effects of fenofibrate on plasma cytokine concentrations in patients with atherosclerosis and hyperlipoproteinemia IIb. Int J Clin Pharmacol Ther 1998; 36: 345–349.
Reddy JK, Azarnoff DL, Hignite CE . Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 1980; 283: 397–398.
Gonzalez FJ . The peroxisome proliferator-activated receptor alpha (PPARalpha): role in hepatocarcinogenesis. Mol Cell Endocrinol 2002; 193: 71–79.
Childs M, Girardot G . [Evaluation of acquired data on long-term risk of hypolipidemic treatments]. Arch Mal Coeur Vaiss 1992; 85(Spec No. 2): 129–133.
Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res 2004; 64: 3849–3854.
Laurora S, Pizzimenti S, Briatore F, Fraioli A, Maggio M, Reffo P et al. Peroxisome proliferator-activated receptor ligands affect growth-related gene expression in human leukemic cells. J Pharmacol Exp Ther 2003; 305: 932–942.
Scatena R, Nocca G, Sole PD, Rumi C, Puggioni P, Remiddi F et al. Bezafibrate as differentiating factor of human myeloid leukemia cells. Cell Death Differ 1999; 6: 781–787.
Liu H, Zang C, Fenner MH, Liu D, Possinger K, Koeffler HP et al. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARalpha/gamma ligand TZD18. Blood 2006; 107: 3683–3692.
Shigeto T, Yokoyama Y, Xin B, Mizunuma H . Peroxisome proliferator-activated receptor alpha and gamma ligands inhibit the growth of human ovarian cancer. Oncol Rep 2007; 18: 833–840.
Muzio G, Maggiora M, Oraldi M, Trombetta A, Canuto RA . PPARalpha and PP2A are involved in the proapoptotic effect of conjugated linoleic acid on human hepatoma cell line SK-HEP-1. Int J Cancer 2007; 121: 2395–2401.
Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA 2008; 105: 985–990.
Swerdlow S, Berger F, Isaacson P, Muller-Hermelink H . Mantle cell lymphoma. In: Harris NL, Jaffe ES, Stein H, Vardiman JW (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2001.
Jares P, Colomer D, Campo E . Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer 2007; 7: 750–762.
Smith MR . Mantle cell lymphoma: advances in biology and therapy. Curr Opin Hematol 2008; 15: 415–421.
Martinez N, Camacho FI, Algara P, Rodriguez A, Dopazo A, Ruiz-Ballesteros E et al. The molecular signature of mantle cell lymphoma reveals multiple signals favoring cell survival. Cancer Res 2003; 63: 8226–8232.
Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ . Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003; 171: 88–95.
Shishodia S, Amin HM, Lai R, Aggarwal BB . Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol 2005; 70: 700–713.
Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ . Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood 2006; 107: 4540–4548.
Tucker CA, Kapanen AI, Chikh G, Hoffman BG, Kyle AH, Wilson IM et al. Silencing Bcl-2 in models of mantle cell lymphoma is associated with decreases in cyclin D1, nuclear factor-kappaB, p53, bax, and p27 levels. Mol Cancer Ther 2008; 7: 749–758.
Rizzatti EG, Falcao RP, Panepucci RA, Proto-Siqueira R, Anselmo-Lima WT, Okamoto OK et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. Br J Haematol 2005; 130: 516–526.
Rizzatti EG, Mora-Jensen H, Weniger MA, Gibellini F, Lee E, Daibata M et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma 2008; 49: 798–808.
Ghielmini M, Zucca E . How I treat mantle cell lymphoma. Blood 2009; 114: 1469–1476.
Amin HM, McDonnell TJ, Medeiros LJ, Rassidakis GZ, Leventaki V, O’Connor SL et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med 2003; 127: 424–431.
Gelebart P, Zak Z, Anand M, Dien-Bard J, Amin HM, Lai R . Interleukin-21 effectively induces apoptosis in mantle cell lymphoma through a STAT1-dependent mechanism. Leukemia 2009; 23: 1836–1846.
Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature 2002; 415: 813–817.
Klier M, Anastasov N, Hermann A, Meindl T, Angermeier D, Raffeld M et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia 2008; 22: 2097–2105.
Gizard F, Nomiyama T, Zhao Y, Findeisen HM, Heywood EB, Jones KL et al. The PPARalpha/p16INK4a pathway inhibits vascular smooth muscle cell proliferation by repressing cell cycle-dependent telomerase activation. Circ Res 2008; 103: 1155–1163.
Zhao X, Li LY . PPAR-alpha agonist fenofibrate induces renal CYP enzymes and reduces blood pressure and glomerular hypertrophy in Zucker diabetic fatty rats. Am J Nephrol 2008; 28: 598–606.
Saidi SA, Holland CM, Charnock-Jones DS, Smith SK . In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Mol Cancer 2006; 5: 13.
Marx N, Sukhova GK, Collins T, Libby P, Plutzky J . PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999; 99: 3125–3131.
Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N . Inhibition of NF-kappaB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin Exp Rheumatol 2005; 23: 323–330.
Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I . Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol 2004; 43: 1481–1488.
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597–608.
Guay DR . Micronized fenofibrate: a new fibric acid hypolipidemic agent. Ann Pharmacother 1999; 33: 1083–1103.
Zambon A, Cusi K . The role of fenofibrate in clinical practice. Diab Vasc Dis Res 2007; 4 (Suppl 3): S15–S20.
Steinhoff M, Beyer M, Roewert-Huber J, Lukowsky A, Assaf C, Sterry W . Complete clinical remission of tumor-stage mycosis fungoides after acute extensive skin necroses, granulomatous reaction, and fever under treatment with bexarotene, vorinostat, and high-dose fenofibrate. J Am Acad Dermatol 2008; 58 (5 Suppl 1): S88–S91.
Krempf M, Rohmer V, Farnier M, Issa-Sayegh M, Corda C, Sirugue I et al. Efficacy and safety of micronised fenofibrate in a randomised double-blind study comparing four doses from 200–400 mg daily with placebo in patients with hypercholesterolemia. Diabetes Metab 2000; 26: 184–191.
Acknowledgements
ZZ and PG are recipients of the Research Fellowship Award of the Alberta Cancer Research Institute. This study is supported by a research operating grant awarded by the National Cancer Institute of Canada to RL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zak, Z., Gelebart, P. & Lai, R. Fenofibrate induces effective apoptosis in mantle cell lymphoma by inhibiting the TNFα/NF-κB signaling axis. Leukemia 24, 1476–1486 (2010). https://doi.org/10.1038/leu.2010.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.117
Keywords
This article is cited by
-
Bcl-2 inhibition combined with PPARα activation synergistically targets leukemic stem cell-like cells in acute myeloid leukemia
Cell Death & Disease (2023)
-
NF-κB signaling and its relevance to the treatment of mantle cell lymphoma
Journal of Hematology & Oncology (2018)
-
Anticancer activity of salicin and fenofibrate
Naunyn-Schmiedeberg's Archives of Pharmacology (2017)
-
Fenofibrate induces apoptosis of triple-negative breast cancer cells via activation of NF-κB pathway
BMC Cancer (2014)
-
Characterization of fenofibrate-mediated anti-proliferative pro-apoptotic effects on high-grade gliomas and anti-invasive effects on glioma stem cells
Journal of Neuro-Oncology (2014)