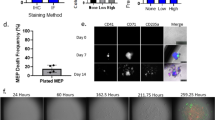
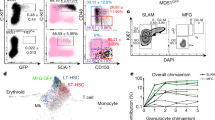
Abstract
A deeper understanding of stem cell niche engagement and subsequent behaviors would be enhanced by technologies enabling the tracking of individual stem cells at the clonal level in long-term co-culture (LTC), which mimics the complexity of the bone marrow microenvironment in vivo. Here, we report the application of time-lapse imaging with intermittent fluorescence for tracking well-defined populations of GFP+ murine hematopoietic stem cells (HSCs) using LTC for >5 weeks. Long-term (LT) and short-term (ST) repopulating HSCs and hematopoietic progenitor cells (HPCs) were compared. The transition from cobblestone areas (CAs) under the stromal cell mantle into dispersed migrating cells on top of the stroma (COS) were directly observed. The ST-HSC and LT-HSC were able to initiate multiple waves of CA formation and COS expansion beyond 2 and 4 weeks, respectively. Retrospective tracking of individual CA forming cell (CAFC) revealed a preference for residing under stroma before the first division and a longer interval before first division for LT-HSC. Inability to maintain quiescence in subsequent divisions was revealed. Our study represents an important starting point from which the LTC system can be augmented to provide a better in vitro model for bone marrow stem cell niches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cheng T . Toward ‘SMART’ stem cells. Gene Ther 2008; 15: 67–73.
Scadden DT . The stem-cell niche as an entity of action. Nature 2006; 441: 1075–1079.
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC . Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996; 183: 1797–1806.
Osawa M, Hanada K, Hamada H, Nakauchi H . Long-term lymphohematopoietic reconstitution by a single CD34− low/negative hematopoietic stem cell. Science 1996; 273: 242–245.
Ema H, Takano H, Sudo K, Nakauchi H . In vitro self-renewal division of hematopoietic stem cells. J Exp Med 2000; 192: 1281–1288.
Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL . The aging of hematopoietic stem cells [see comments]. Nat Med 1996; 2: 1011–1016.
Wagers AJ, Weissman IL . Differential expression of alpha2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1+ hematopoietic stem cells. Stem Cells 2006; 24: 1087–1094.
Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G . In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med 2003; 9: 1428–1432.
Bianco P, Robey PG, Simmons PJ . Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008; 2: 313–319.
Dexter TM, Allen TD, Lajtha LG . Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol 1977; 91: 335–344.
Dexter TM, Moore MA, Sheridan AP . Maintenance of hemopoietic stem cells and production of differentiated progeny in allogeneic and semiallogeneic bone marrow chimeras in vitro. J Exp Med 1977; 145: 1612–1616.
Schofield R . The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4: 7–25.
Frimberger AE, Stering AI, Quesenberry PJ . Characterization of engraftable hematopoietic stem cells in murine long-term bone marrow cultures. Exp Hematol 2001; 29: 643–652.
Moore KA, Lemischka IR . Stem cells and their niches. Science 2006; 311: 1880–1885.
Frimberger AE, McAuliffe CI, Werme KA, Tuft RA, Fogarty KE, Benoit BO et al. The fleet feet of haematopoietic stem cells: rapid motility, interaction and proteopodia. Br J Haematol 2001; 112: 644–654.
Wagner W, Saffrich R, Wirkner U, Eckstein V, Blake J, Ansorge A et al. Hematopoietic progenitor cells and cellular microenvironment: behavioral and molecular changes upon interaction. Stem Cells 2005; 23: 1180–1191.
Rieger MA, Schroeder T . Exploring hematopoiesis at single cell resolution. Cells Tissues Organs 2008; 188: 139–149.
Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell 2007; 1: 541–554.
Ploemacher RE, van der Sluijs JP, van Beurden CA, Baert MR, Chan PL . Use of limiting-dilution type long-term marrow cultures in frequency analysis of marrow-repopulating and spleen colony-forming hematopoietic stem cells in the mouse. Blood 1991; 78: 2527–2533.
de Haan G, Van Zant G . Intrinsic and extrinsic control of hemopoietic stem cell numbers: mapping of a stem cell gene. J Exp Med 1997; 186: 529–536.
Absher PM, Absher RG . Clonal variation and aging of diploid fibroblasts. Cinematographic studies of cell pedigrees. Exp Cell Res 1976; 103: 247–255.
Nadarajah B, Alifragis P, Wong RO, Parnavelas JG . Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex 2003; 13: 607–611.
DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA . Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol 1993; 122: 729–737.
Dykstra B, Ramunas J, Kent D, McCaffrey L, Szumsky E, Kelly L et al. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc Natl Acad Sci USA 2006; 103: 8185–8190.
Frimberger AE, Stering AI, Quesenberry PJ . An in vitro model of hematopoietic stem cell homing demonstrates rapid homing and maintenance of engraftable stem cells. Blood 2001; 98: 1012–1018.
Neumann B, Held M, Liebel U, Erfle H, Rogers P, Pepperkok R et al. High-throughput RNAi screening by time-lapse imaging of live human cells. Nat Methods 2006; 3: 385–390.
Schroeder T . Tracking hematopoiesis at the single cell level. Ann NY Acad Sci 2005; 1044: 201–209.
Bahnson A, Athanassiou C, Koebler D, Qian L, Shun T, Shields D et al. Automated measurement of cell motility and proliferation. BMC Cell Biol 2005; 6: 19.
Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T . In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol 2004; 6: 436–442.
Takano H, Ema H, Sudo K, Nakauchi H . Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med 2004; 199: 295–302.
van der Sluijs JP, de Jong JP, Brons NH, Ploemacher RE . Marrow repopulating cells, but not CFU-S, establish long-term in vitro hemopoiesis on a marrow-derived stromal layer. Exp Hematol 1990; 18: 893–896.
Breems DA, Blokland EA, Siebel KE, Mayen AE, Engels LJ, Ploemacher RE . Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood 1998; 91: 111–117.
Ploemacher RE, van der Sluijs JP, Voerman JS, Brons NH . An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood 1989; 74: 2755–2763.
Gorbunov NV, Pogue-Geile KL, Epperly MW, Bigbee WL, Draviam R, Day BW et al. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat Res 2000; 154: 73–86.
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD . Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 2007; 104: 5431–5436.
Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 2008; 135: 1118–1129.
Kohler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 2009; 114: 290–298.
Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 2009; 457: 97–101.
Acknowledgements
We thank Dr Sallie Boggs for helpful discussion and stimulating suggestions, and Julie Goff for help collecting data. We thank Charalambos Athanassiou and Lei Qian for maintaining the automatic system and for statistical analysis. This investigation was supported by the NIH grants RO1 EB 001051 (to RH), R01 AI080424 and RO1 HL 075601 (to TC). TC was a recipient of the Scholar Award from the Leukemia & Lymphoma Society (1027-09), a recipient of the Changjiang Scholarship from the Ministry of Education of China (2007-JGT-08) and a recipient of the Outstanding Young Scholar Award from the National Natural Science Foundation of China (30825017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Song, Y., Bahnson, A., Hall, N. et al. Stem cell traits in long-term co-culture revealed by time-lapse imaging. Leukemia 24, 153–161 (2010). https://doi.org/10.1038/leu.2009.191
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2009.191
Keywords
This article is cited by
-
Evaluation of committed and primitive cord blood progenitors after expansion on adipose stromal cells
Cell and Tissue Research (2018)
-
Altered mesenchymal niche cells impede generation of normal hematopoietic progenitor cells in leukemic bone marrow
Leukemia (2016)
-
Label-free imaging to study phenotypic behavioural traits of cells in complex co-cultures
Scientific Reports (2016)
-
Apoptosis, DAP-Kinase1 Expression and the Influences of Cytokine Milieu and Mesenchymal Stromal Cells on Ex Vivo Expansion of Umbilical Cord Blood-Derived Hematopoietic Stem Cells
Indian Journal of Hematology and Blood Transfusion (2016)
-
Small-molecule inhibitors targeting INK4 protein p18INK4C enhance ex vivo expansion of haematopoietic stem cells
Nature Communications (2015)