Abstract
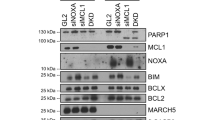
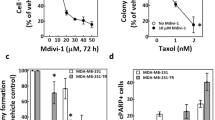
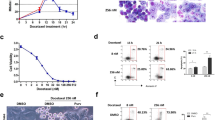
Kinesin spindle protein (KSP), a microtubule-associated motor protein essential for cell cycle progression, is overexpressed in many cancers and is a potential anti-tumor target. We found that inhibition of KSP by a selective inhibitor, ARRY-520, blocked cell cycle progression, leading to apoptosis in acute myeloid leukemia cell lines that express high levels of KSP. Knockdown of p53, overexpression of XIAP and mutation in caspase-8 did not significantly affect sensitivity to ARRY-520, suggesting that the response is independent of p53, XIAP and the extrinsic apoptotic pathway. Although ARRY-520 induced mitotic arrest in both HL-60 and Bcl-2-overexpressing HL-60Bcl-2 cells, cell death was blunted in HL-60Bcl-2 cells, suggesting that the apoptotic program is executed through the mitochondrial pathway. Accordingly, inhibition of Bcl-2 by ABT-737 was synergistic with ARRY-520 in HL-60Bcl-2 cells. Furthermore, ARRY-520 increased Bim protein levels prior to caspase activation in HL-60 cells. ARRY-520 significantly inhibited tumor growth of xenografts in SCID mice and inhibited AML blast but not normal colony formation, supporting a critical role for KSP in proliferation of leukemic progenitor cells. These results demonstrate that ARRY-520 potently induces cell cycle block and subsequent death in leukemic cells via the mitochondrial pathway and has the potential to eradicate AML progenitor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Jordan MA, Wilson L . Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253–265.
Goodson HV, Kang SJ, Endow SA . Molecular phylogeny of the kinesin family of microtubule motor proteins. J Cell Sci 1994; 107: 1875–1884.
Walczak CE, Mitchison TJ . Kinesin-related proteins at mitotic spindle poles: function and regulation. Cell 1996; 85: 943–946.
Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA . Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 1995; 83: 1159–1169.
Valentine MT, Fordyce PM, Block SM . Eg5 steps it up!. Cell Div 2006; 1: 31.
Hansen GM, Justice MJ . Activation of Hex and mEg5 by retroviral insertion may contribute to mouse B-cell leukemia. Oncogene 1999; 18: 6531–6539.
Kaiser A, Brembeck FH, Nicke B, Wiedenmann B, Riecken EO, Rosewicz S . All-trans-retinoic acid-mediated growth inhibition involves inhibition of human kinesin-related protein HsEg5. J Biol Chem 1999; 274: 18925–18931.
Koller E, Propp SS, Hirsch S, Shepard PJ, Dean NM . Inhibition of Eg5 (Kinesin-like 1) with antisense oligonucleotide leads to arrest in G2/M of cell cycle and shows potent antitumor activity against a variety of tumors. Clin Cancer Res 2003; 9: 6221S.
Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, Skorski T . Chronic myelogenous leukemia molecular signature. Oncogene 2003; 22: 3952–3963.
Carter BZ, Mak DH, Shi Y, Schober WD, Wang RY, Konopleva M et al. Regulation and targeting of Eg5, a mitotic motor protein in blast crisis CML: overcoming imatinib resistance. Cell Cycle 2006; 5: 2223–2229.
Burris H, LoRusso P, Jones S, McCormick J, Willcutt N, Hodge J et al. A phase I study to determine the safety and pharmacokinetics of intravenous administration of SB715992 on a once weekly for three consecutive weeks schedule in patients with refractory solid tumors. Eur J Cancer 2003; 1: S172–S173.
Williams D, Kathman S, Chu Q, Holen K, Rowinsky E, Wilding G et al. A phase I study of SB-715992, a novel kinesin spindle protein (KSP) inhibitor: pharmacokinetic (PK)/pharmacodynamic (PD) modeling of absolute neutrophil counts (ANC). Eur J Cancer 2008; 2: 20–21.
Chu Q, Holen KD, Rowinsky EK, Alberti DB, Monroe P, Volkman JL et al. A phase I study to determine the safety and pharmacokinetics of IV administered SB-715992, a novel kinesin spindle protein (KSP) inhibitor, in patients (pts) with solid tumors. Proc Am Soc Clin Oncol 2003; 22: 131 (abstract 525).
Holen KD, Belani CP, Wilding G, Ramalingam S, Volkman JL, Ramanathan RK et al. Phase I study to determine tolerability and pharmacokinetics (PK) of SB-743921, a novel kinesin spindle protein (KSP) inhibitor. J Clin Oncol 2006; 24: 79S.
Miglarese MR, Carlson RO . Development of new cancer therapeutic agents targeting mitosis. Expert Opin Investig Drugs 2006; 15: 1411–1425.
Lemieux C, DeWolf W, Voegtli W, DeLisle RK, Laird E, Wallace E et al. ARRY-520: a novel, highly selective KSP inhibitor with potent anti-proliferative activity. AACR Annual Meeting, 2007.
Woessner RD, Corrette C, Allen S, Hans J, Zhao Q, Aicher T et al. ARRY-520, a KSP inhibitor with efficacy and pharmacodynamic activity in animal models of solid tumors. AACR Meet Abstr 2007: 1433.
Juo P, Kuo CJ, Yuan J, Blenis J . Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol 1998; 8: 1001–1008.
Carter BZ, Mak DH, Schober WD, Dietrich MF, Pinilla C, Vassilev LT et al. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood 2008; 111: 3742–3750.
Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D . XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J Biol Chem 2000; 275: 31733–31738.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Martin S, Reutelingsperger C, McGahon A, Rader J, van Schie R, LaFace D et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and ABL. J Exp Med 1995; 182: 1545–1556.
Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia 2003; 17: 2081–2089.
Estrov Z, Black RA, Sleath PR, Harris D, Van Q, LaPushin R et al. Effect of interleukin-1 beta converting enzyme inhibitor on acute myelogenous leukemia progenitor proliferation. Blood 1995; 86: 4594–4602.
Chou TC, Talalay P . Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55.
Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE et al. p53 status and the efficacy of cancer therapy in vivo. Science 1994; 266: 807–810.
Tan G, Heqing L, Jiangbo C, Ming J, Yanhong M, Xianghe L et al. Apoptosis induced by low-dose paclitaxel is associated with p53 upregulation in nasopharyngeal carcinoma cells. Int J Cancer 2002; 97: 168–172.
Esteve MA, Carre M, Braguer D . Microtubules in apoptosis induction: are they necessary? Curr Cancer Drug Targets 2007; 7: 713–729.
Goncalves A, Braguer D, Carles G, Andre N, Prevot C, Briand C . Caspase-8 activation independent of CD95/CD95-L interaction during paclitaxel-induced apoptosis in human colon cancer cells (HT29-D4). Biochem Pharmacol 2000; 60: 1579–1584.
Kim R, Tanabe K, Emi M, Uchida Y, Toge T . Death receptor-dependent and -independent pathways in anticancer drug-induced apoptosis of breast cancer cells. Oncol Rep 2003; 10: 1925–1930.
Yamaguchi H, Chen J, Bhalla K, Wang HG . Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem 2004; 279: 39431–39437.
Tudor G, Aguilera A, Halverson DO, Laing ND, Sausville EA . Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ 2000; 7: 574–586.
Li R, Moudgil T, Ross HJ, Hu HM . Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ 2005; 12: 292–303.
Esteve MA, Carre M, Braguer D . Microtubules in apoptosis induction: are they necessary? Curr Cancer Drug Targets 2007; 7: 713–729.
Gilley J, Coffer PJ, Ham J . FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol 2003; 162: 613–622.
Huang H, Regan KM, Lou Z, Chen J, Tindall DJ . CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 2006; 314: 294–297.
Tao W, South VJ, Diehl RE, Davide JP, Sepp-Lorenzino L, Fraley ME et al. An inhibitor of the kinesin spindle protein activates the intrinsic apoptotic pathway independently of p53 and de novo protein synthesis. Mol Cell Biol 2007; 27: 689–698.
Acknowledgements
We thank Elena S Vess and Betty Notzon for helping with the preparation of the paper and Wenjing Chen for helping with collecting patient information. This work was supported in part by grants from the National Institutes of Health (P01 CA49639, P01 CA55164, and CA16672) to MA and Array BioPharma to BZC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Carter, B., Mak, D., Woessner, R. et al. Inhibition of KSP by ARRY-520 induces cell cycle block and cell death via the mitochondrial pathway in AML cells. Leukemia 23, 1755–1762 (2009). https://doi.org/10.1038/leu.2009.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2009.101
Keywords
This article is cited by
-
NAT10 regulates mitotic cell fate by acetylating Eg5 to control bipolar spindle assembly and chromosome segregation
Cell Death & Differentiation (2022)
-
Thiadiazole derivatives as anticancer agents
Pharmacological Reports (2020)
-
CMPD1 inhibited human gastric cancer cell proliferation by inducing apoptosis and G2/M cell cycle arrest
Biological Research (2018)
-
Sulforaphane potentiates growth-inhibiting and apoptosis-promoting activities of cisplatin following oxidative stress and mitochondrial dysfunction in malignant mesothelioma cells
Molecular & Cellular Toxicology (2016)
-
Impact of kinesin Eg5 inhibition by 3,4-dihydropyrimidin-2(1H)-one derivatives on various breast cancer cell features
BMC Cancer (2015)