Abstract
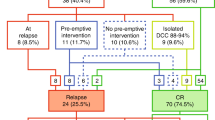
Treatment response is a strong outcome predictor for childhood acute lymphoblastic leukemia (ALL). Here, we evaluated the predictive impact of flow cytometric blast quantification assays (absolute blast count, BC, and blast reduction rate, BRR) in peripheral blood (pB) and/or bone marrow (BM) at early time points of induction therapy (days 0, 8 and 15) on the remission status in the AIEOP-BFM-ALL 2000 protocol. At the single parameter level (905 patients), the strongest predictive parameter for the remission status as a dichotomous minimal residual disease (MRD) parameter (positive/negative) has been provided by the BC at day 15 in BM (cutoff: 17 blasts/μl; 50 vs 15%; odds ratio: 5.6; 95% confidence interval: 4.1–7.6, P<0.001), followed by the BRR at day 15 in BM and by the BC at day 8 in pB (odds ratios: 3.8 and 2.6, respectively). In the multiple regression analysis (440 patients), BC in pB (d0 and d8) and in BM (d15) as well as BRR at day 8 in pB provided significantly contributing variables with an overall correct prediction rate of 74.8%. These data show that the quantitative assessment of early response parameters, especially absolute BCs at day 15 in BM, has a predictive impact on the remission status after induction therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kamps WA, Veerman AJ, van Wering ER, van Weerden JF, Slater R, van der Does-van den Berg A . Long-term follow-up of Dutch Childhood Leukemia Study Group (DCLSG) protocols for children with acute lymphoblastic leukemia, 1984–1991. Leukemia 2000; 14: 2240–2246.
Maloney KW, Shuster JJ, Murphy S, Pullen J, Camitta BA . Long-term results of treatment studies for childhood acute lymphoblastic leukemia: Pediatric Oncology Group studies from 1986–1994. Leukemia 2000; 14: 2276–2285.
Pui CH, Boyett JM, Rivera GK, Hancock ML, Sandlund JT, Ribeiro RC et al. Long-term results of total therapy studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children's Research Hospital. Leukemia 2000; 14: 2286–2294.
Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia 2000; 14: 2205–2222.
Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood 2002; 100: 52–58.
Flohr T, Schrauder A, Cazzaniga G, Panzer-Grumayer R, van der Velden V, Fischer S et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia 2008; 22: 771–782.
Riehm H, Reiter A, Schrappe M, Berthold F, Dopfer R, Gerein V et al. [Corticosteroid-dependent reduction of leukocyte count in blood as a prognostic factor in acute lymphoblastic leukemia in childhood (therapy study ALL-BFM 83)]. Klin Padiatr 1987; 199: 151–160.
Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 2000; 95: 3310–3322.
Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH et al. Cytoreduction and prognosis in acute lymphoblastic leukemia—the importance of early marrow response: report from the Children's Cancer Group. J Clin Oncol 1996; 14: 389–398.
Cazzaniga G, Biondi A . Molecular monitoring of childhood acute lymphoblastic leukemia using antigen receptor gene rearrangements and quantitative polymerase chain reaction technology. Haematologica 2005; 90: 382–390.
Szczepanski T, Flohr T, van der Velden VH, Bartram CR, van Dongen JJ . Molecular monitoring of residual disease using antigen receptor genes in childhood acute lymphoblastic leukaemia. Best Pract Res Clin Haematol 2002; 15: 37–57.
Dworzak MN, Gaipa G, Ratei R, Veltroni M, Schumich A, Maglia O et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: Multi-centric assessment is feasible. Results of the AIEOP-BFM-ALL-FCM-MRD-Study Group. Cytometry B Clin Cytom 2008; 74: 331–340.
Bonney GE . Logistic regression for dependent binary observations. Biometrics 1987; 43: 951–973.
Demidenko E . Sample size and optimal design for logistic regression with binary interaction. Stat Med 2008; 27: 36–46.
Griner PF, Mayewski RJ, Mushlin AI, Greenland P . Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med 1981; 94: 557–592.
Zweig MH, Campbell G . Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561–577.
Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood 2002; 99: 1952–1958.
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 2008; 111: 5477–5485.
Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer 1997; 80: 1717–1726.
Gadner H, Masera G, Schrappe M, Eden T, Benoit Y, Harrison C et al. The Eighth International Childhood Acute Lymphoblastic Leukemia Workshop (‘Ponte di legno meeting’) report, Vienna, Austria, April 27–28, 2005. Leukemia 2006; 20: 9–17.
Schrappe M . Prognostic factors in childhood acute lymphoblastic leukemia. Indian J Pediatr 2003; 70: 817–824.
Acknowledgements
We thank all participants and institutions of the AIEOP-BFM-ALL 2000 trial for their close cooperation in providing samples and data from their patients. We are also grateful to M Dunken (Berlin), B Oestereich (Berlin), A Schumich (Vienna), G Froschl (Vienna), Z Husak (Vienna) and O Maglia (Monza) for excellent technical assistance and C Feiler (Berlin) for data management. This work was supported by Wilhelm Sander Stiftung (Grant no. 2004.072.1). Peter Rhein was supported by Deutsche José Carreras Leukämie-Stiftung (Grant no. DJCLS-F05/09). Michael Dworzak was supported by the Austrian National Bank (Grant no. 10962) and Giuseppe Gaipa was supported by Fondazione Tettamanti and Fondazione Cariplo.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ratei, R., Basso, G., Dworzak, M. et al. Monitoring treatment response of childhood precursor B-cell acute lymphoblastic leukemia in the AIEOP-BFM-ALL 2000 protocol with multiparameter flow cytometry: predictive impact of early blast reduction on the remission status after induction. Leukemia 23, 528–534 (2009). https://doi.org/10.1038/leu.2008.324
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.324
Keywords
This article is cited by
-
Measurable residual disease (MRD)-testing in haematological and solid cancers
Leukemia (2024)
-
Treatment of higher risk acute lymphoblastic leukemia in young people (CCG-1961), long-term follow-up: a report from the Children’s Oncology Group
Leukemia (2019)
-
Correlation of Day 8 Steroid Response with Bone Marrow Status Measured on Days 14 and 35, in Patients with Acute Lymphoblastic Leukemia Being Treated with BFM Protocol
Indian Journal of Hematology and Blood Transfusion (2019)
-
Role of peripheral blood minimum residual disease at day 8 of induction therapy in high-risk pediatric patients with acute lymphocytic leukemia
Scientific Reports (2016)
-
Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells
Journal of Translational Medicine (2011)