Abstract
Abnormal regulation of cell migration and altered rearrangement of the cytoskeleton are fundamental properties of metastatic cells. The first identified metastasis suppressor NM23-H1, which displays nucleoside-diphosphate kinase (NDPK) activity is involved in these processes. NM23-H1 inhibits the migratory and invasive potential of some cancer cells. Correspondingly, numerous invasive cancer cell lines (eg, breast, colon, oral, hepatocellular carcinoma, and melanoma) display low endogenous NM23 levels. In this review, we summarize mechanisms, which are linked to the anti-metastatic activity of NM23. In human cancer cell lines NM23-H1 was shown to regulate cytoskeleton dynamics through inactivation of Rho/Rac-type GTPases. The Drosophila melanogaster NM23 homolog abnormal wing disc (AWD) controls tracheal and border cell migration. The molecular function of AWD is well characterized in both processes as a GTP supplier of Shi/Dynamin whereby AWD regulates the level of chemotactic receptors on the surface of migrating cells through receptor internalization, by its endocytic function. Our group studied the role of the sole group I NDPK, NDK-1 in distal tip cell (DTC) migration in Caenorhabditis elegans. In the absence of NDK-1 the migration of DTCs is incomplete. A half dosage of NDPK as present in ndk-1 (+/−) heterozygotes results in extra turns and overshoots of migrating gonad arms. Conversely, an elevated NDPK level also leads to incomplete gonadal migration owing to a premature stop of DTCs in the third phase of migration, where NDK-1 acts. We propose that NDK-1 exerts a dosage-dependent effect on the migration of DTCs. Our data derived from DTC migration in C. elegans is consistent with data on AWD’s function in Drosophila. The combined data suggest that NDPK enzymes control the availability of surface receptors to regulate cell-sensing cues during cell migration. The dosage of NDPKs may be a coupling factor in cell migration by modulating the efficiency of receptor recycling.
Similar content being viewed by others
Main
Metastasis suppressors inhibit different steps of metastasis formation without globally influencing primary tumor growth. Current knowledge suggests that the human genome contains ~30 genes encoding proteins displaying metastasis suppressor activity.1
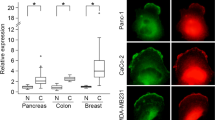
Metastasis inhibitors are either not, or poorly expressed in metastatic colonies. NM23-M1 (non-metastatic clone 23, mouse isoform 1) was identified as the first metastasis suppressor ~30 years ago by Patricia Steeg by comparing expression patterns of invasive and non-invasive mouse melanoma cell lines.2 Although expression of NM23-H1, the human counterpart of NM23-M1 was found to be elevated in primary tumors, downregulation of NM23-H1 expression was observed in multiple examples of metastatic tumors, such as breast, hepatocellular, colon cancer, and melanoma.3, 4, 5 However, it is important to note that in several cancer types (for example, cervical, ovarian, prostate tumors, or hematologic malignancies) positive association was reported between NM23-H1 expression and tumor progression.6, 7, 8, 9
NM23 (or also called NME, which stands for non-metastatic) gene family members encode nucleoside-diphosphate kinases (NDPKs), which were described >50 years ago10 as housekeeping enzymes meriting a few lines of text in most biochemistry journals as ’just’ catalyzing the conversion of nucleoside diphosphates to nucleoside triphospates. Yet, the human genome consists of 10 NM23 paralogs, which are grouped based on their sequence similarity and NDPK activity. Group I NDPKs (NM23-H1-H4 isoforms) all possess NDPK activity and show high sequence homology to one another, whereas group II homologs (NM23-H5-H9 and retinitis pigmentosa 2 (RP2)) are more divergent in sequence and do not display the above enzymatic activity except for the H6 isoform.11 NDPKs function as homo- and/or heterohexamers, assembled from H1 and/or H2 monomers (reviewed in reference 12). However, in some instances (see Muimo review in this series), mixed hexamers do not appear to form when associated with other proteins in the membrane such as the cystic fibrosis protein, CFTR.
NM23 homologs are linked to numerous biological processes such as cell proliferation, differentiation,13, 14 cell migration,15 signal transduction, transcriptional regulation,16, 17 apoptosis,18 and many aspects of development (reviewed in reference 19).
Besides NDPK activity some other molecular activities have been attributed to NDPKs, including histidine-dependent protein kinase (histidine phosphotransferase),20, 21 nuclease activity,22, 23 and lipid bilayer binding.24, 25, 26, 27, 28
NM23-H1 is known to inhibit the migratory and invasive potential of cancer cells. Although the exact molecular mechanisms underlying these processes remain elusive, several mechanisms are consistent with the anti-metastatic activity of NM23. In this work, we briefly summarize the mechanisms whereby NM23 or its homologs influence the migratory potential of cells. First we focus on cell lines, then move to the function of the Drosophila melanogaster NM23 homolog abnormal wing disc (AWD) in tracheal and border cell migration. Finally, our data derived from distal tip cell (DTC) migration in the model organism Caenorhabditis elegans are compared, first of all with AWD’s function in Drosophila and then with human data.
Nm23-h1 efficiently inhibits the migratory and invasive potential of tumor cells
Among many functions ascribed to NM23-H1, its ability to suppress motility and invasiveness of tumor cells is a well-accepted characteristics.29 The anti-metastatic effect of NM23-H1 (and its mouse homolog NM23-M1) was demonstrated using mouse models and different cancer cell lines. Boissan et al30 showed that crossing of nm23-M1 knockout mice with a mouse strain in which hepatocellular carcinoma had been induced, resulted in double transgenic mice with a higher incidence of lung metastases. The metastasis-suppressive function of NM23 was also confirmed by overexpressing NM23-M1 in highly metastatic K-1735 melanoma cells, where endogenous NM23 expression was low: ectopic expression of NM23 therein resulted in reduction in their metastatic potential.31 Similar results were obtained when invasive breast, colon, oral, and hepatocellular carcinoma and different melanoma cell lines, displaying low endogenous NM23 levels were transfected with transgenes encoding the H1 isoform.32, 33, 34, 35, 36, 37
Conversely, silencing of NM23 in non-invasive hepatocellular carcinoma and colon cancer cell lines possessing substantial or high endogenous NM23-H1 levels, led to a metastatic, invasive phenotype by altering cell–cell contacts, migratory potential and major signaling pathways linked to tumor progression. As a consequence of NM23 silencing Boissan et al38 observed upregulation of MT1-MMP (membrane-associated matrix metalloproteinase), increased Rac1 signaling and activation of MAPK (mitogen-activated protein kinase)/SAPK (stress-activated protein kinases) and Akt pathways. Although the exact molecular mechanism whereby NM23 regulates cell migration still remains unclear, the above data suggest that NM23-H1 inhibits the activity of Rac1, a pleiotropic regulator of cell motility. Indeed, NM23-H1 was shown to negatively regulate a Rac1-specific nucleotide exchange factor, Tiam1, thereby inhibiting Rac1 activation.39 NM23-H1 was also connected to Rac-Rho activation by reducing transcription of the EDG2 gene, encoding a lysophosphatidic acid receptor.40, 41 Dbl-1, a specific exchange factor of another Rho-type GTPase, Cdc42, was also identified as a binding partner of NM23-H1. Binding of NM23-H1 to Dbl-1 resulted in inactivation of Cdc42.42
The role of the drosophila ndpk homolog awd in the regulation of tracheal morphogenesis and border cell migration
The function of NDPKs in cell migration was best characterized in vivo in D. melanogester. The NDPK homolog AWD shows 78% sequence identity to NM23-H1 and H2 isoforms.43 AWD regulates negatively the migration of tracheal and border cells, by influencing the endocytosis of certain chemotactic receptors driving the above mentioned processes.44, 45
Tracheal development in Drosophila is used to model tubular morphogenesis (also termed branching morphogenesis (reviewed in reference 46, 47, see also Muimo in this series of papers). Tracheogenesis starts during early stages of embryogenesis when tracheal placodes are formed through the invagination of specialized ectodermal cells. From these placodes develop the tracheal branches, which then elongate and finally fuse to shape the tracheal network. Branching morphogenesis is interpreted as a series of cell migration events as cell division is completed after placode formation. Fibroblast growth factor/fibroblast growth factor receptor (FGF/FGFR) signaling has a crucial role in tracheal migration. The ligand, branchless/FGF, which is released by the tissues ahead of the advancing tube, is recognized by breathless/FGFR expressed on the surface of migrating tracheal cells. AWD influences FGFR levels on these advancing tracheal cell surfaces through recycling of the FGF receptor. Endocytosis of FGFR is regulated by Shibire/Dynamin, an atypical large GTPase, whose GTP supply is mediated by AWD.44 Mechanistically, it was recently shown by Boissan et al48 that NDPKs fuel Dynamin type GTPases locally by GTP to allow them to work at high thermodynamic efficiency.
Interestingly, homozygous (awd+/awd−) loss-of-function mutants and awd+/awd− heterozygotes show different phenotypes such that in homozygous individuals, a complete disruption of tracheal tubules with dispersed tracheal cells was noted, whereas in heterozygotes ectopic branch migration was observed.49 We will return to this theme in relation to cystic fibrosis later in the review.
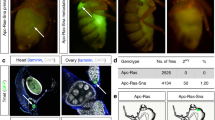
Border cell migration during Drosophila oogenesis is an important model of vectorial epithelial cell migration.50 The Drosophila egg chamber contains the germ cell complex (oocyte and the nurse cells) surrounded by follicular (epithelial) cells. During oogenesis AWD is expressed in the follicular cells. Border cells—which are a special group of 6–10 epithelial follicular cells—secede from the epithelium and migrate towards the anterior pole of the oocyte, and as a result they form the micropyle.50 AWD is expressed in the follicular epithelial cells, but its expression is downregulated in the border cells, which allows their migration.45 In contrast, overexpressing AWD in the border cells blocks their motility.45 Among other pathways platelet derived growth factor receptor (PDGF) and JAK/STAT signaling are known to drive border cell migration.51, 52, 53 AWD regulates the migration of border cells through affecting receptor levels on the cell surface via internalization of the vascular endothelial growth factor receptor (VEGFR)/PDGFR homolog Pvr and the JAK/STAT receptor homolog Domeless in cooperation with Shi/Dynamin.45, 51, 52 The mechanism whereby AWD regulates Pvr and Domeless receptor levels on border cell surfaces is similar to that in tracheogenesis (see above). Thus we have an emerging paradigm that a key regulator of nucleotide balance is also regulating receptor residence time.
The idea of ndpk’s dosage dependence comes from drosophila: awd functions in the follicular epithelial cells of the egg chamber in a dosage-dependent manner
The Shearn laboratory showed first that AWD is expressed and functions in the follicular cells of the egg chamber during oogenesis.54 Next, the Hsu laboratory demonstrated that AWD is required to maintain the epithelial integrity of follicular cells by regulating the turnover of adherens junction components.55 AWD exerts this effect through its endocytic controlling function toward multiple adherens junction components such as E-cadherin, beta-catenin, and alpha-spectrin.
In addition, Hsu and colleagues examined how lack and overexpression of AWD affect the morphology and structure of follicular epithelial cells. The absence of AWD in these cells resulted in an abnormal epithelial structure: because of spreading of adherens junction components, the cells accumulated and piled up. On the other hand, as a consequence of excess AWD follicular cells lose adherens junction components from their surface, adopted a spindle-like morphology and underwent a morphological change reminiscent of EMT.56 These data first showed that both lack or have an excess AWD activity disturb epithelial integrity and suggested that an optimal dosage of AWD is needed to balance demand and supply of adherens junction components in follicular cells.49
The dosage-dependent effect of ndk-1 exerted on dtc migration in c. elegans
Consequently, we investigated the potential dosage-dependent effect of NDK-1, the nematode group I homolog NDPK57 exerted on cell migration, especially on the migration of DTCs in Caenorhabditis elegans.
NDK-1, the sole C. elegans group I NDPK, shows 65% identity and 85% overall similarity to NM23-H1 and H2 isoforms.57 As group I NDPKs are well-known negative regulators of cell migration, first we intended to examine how the conserved worm protein, NDK-1 functions in a human environment, using highly invasive MDA-MB231T breast carcinoma cells as a model system, where endogenous NM23 level is low.40 Therefore, MDA-MB231T cells were transfected with worm NDK-1, NM23-H1 and H2, respectively, and the motility of the transfected lines was examined.58 We found that the migratory potential of MDA-MB231T cells was significantly suppressed, when NDK-1 or its human counterparts were overexpressed. These data show that NDK-1 acts in a conserved manner in cell migration and that C. elegans serves as a tractable genetic model to study the biological functions of NDPKs.
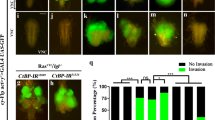
Caenorhabditis elegans serves as a useful and simple model system to investigate the process of cell migration. DTCs are responsible for gonadal morphogenesis (reviewed in reference 59): these cells of somatic gonadal origin generate the shape of the gonad. DTCs are born in L1 larval stage and are located at the distal edges of the gonad primordium (Figure 1a). In L2 stage one of the two DTCs moves anteriorly, the other posteriorly along the ventral body wall muscles, resulting in an elongated gonad. In L3 larval stage each DTC turns 90 degrees and moves to the dorsal muscles. After a second turn DTCs migrate dorsally to the midbody region in L4 larvae and finish their migration in adulthood dorsal to the vulva. In adult hermaphrodites this migratory path results in two symmetric U-shaped gonad arms,59, 60 which can be easily followed during development by DIC microscopy owing to transparency of the worm (Figure 1b).
(a) Schematic review of DTC migration (based on Fancsalszky et al, 2014). DTCs are present on the distal ends of the gonad primordium in the L1 larval stage. In L1 and L2 larval stages the DTCs migrate along the ventral body muscles in response to attractive cues (first or ventral phase of migration). In the figure ventral body wall muscle is symbolized by a dashed line. In L3 stage distal tip cells turn towards the dorsal side (second or ventral to dorsal phase of migration), finally they turn again in L4 stage and migrate further proximally, in adulthood by reaching their final position opposite to the vulva (third or dorsal phase of migration). Developmental stages are indicated at the right of the figure. The different levels of NDK-1 influence DTC migration. (b) The precise dosage of ndk-1 is needed for the development of wild-type (N2) U-shaped gonad arms. Changes in the dosage of ndk-1 lead to different DTC migration phenotypes. (c) In ndk-1(+/−) heterozygotes we detected ectopically migrating DTCs (in this panel extra turn phenotype) with 24% of penetrance. (d) In ndk-1(−/−) homozygotes majority of the animals showed reduced migration, resulting in J-shaped gonad arms. (e) If we overexpressed NDK-1, incomplete migration was observed as well, however only in 14% of the cases due to mozaicism of the transgene. Scalebars are 100 μm, arrows show the position of the vulva, dotted lines follow the path of the DTCs, resulting the shape of the gonad.
Hence, we examined the role of NDK-1 in gonadal migration in the worm. First we characterized homozygous ndk-1(−/−) loss-of-function mutants and found that these nematodes display an abnormal gonad shape. In addition, worms transgenic for NDK-1::GFP showed expression in the DTCs. The above data indicated that NDK-1/NDPK plays a role in DTC migration. Detailed analysis of homozygous ndk-1(−) mutants revealed that in the absence of NDK-1 the migration of DTCs is incomplete, results in J-shaped gonad arms in the majority of mutants due to a premature stop of DTCs in the third (dorsal) phase of migration;58 (Figure 1d).
This finding was interesting but perhaps surprising, because by knocking out a group I NDPK homolog we would expect enhanced/ectopic migration of DTCs instead of incompletely developed gonad arms, based on the well-known inhibitory effect exerted on cell migration by this gene family. However, overexpression of NDK-1 in highly invasive breast carcinoma cells caused inhibition of the migratory potential similar to NM23-H1 and H2. One possible explanation would be that NDK-1 affects cell migration in opposing ways in different cellular environments (eg, it promotes migration of DTCs in the worm but inhibits cell migration in human cell lines) similar to Dyctiostelium, where NDPK was shown to influence growth in an opposing manner depending on a spatial cellular context of axenic growth in broth versus spreading growth on a surface.61
To better understand NDK-1’s function in the worm, we further investigated how different levels of NDK-1 expression affect the migration of DTCs: in order to complete our data set we analyzed DTC phenotypes in ndk-1(+/−) heterozygotes and NDK-1-overexpressing transgenic worms.
In outwardly normal wild-type ndk-1(+/−) heterozygotes, a half dosage of NDK-1 protein is expressed. In the majority of heterozygous worms the reduced level of NDK-1 did not interfere with gonad morphogenesis, as in 76% of the cases wild-type gonad arms were observed (n=66). However, ectopically migrating DTCs were found in 24% of the observed gonad arms (n=66), indicating that in these worms one single functional copy of the ndk-1 gene does not produce sufficient NDK-1 protein to generate wild-type U-shaped gonad arms (haploinsufficiency with 24% penetrance). Ectopically migrating gonad arms manifested either in an overshoot (5%) or an extra turn (19%) phenotype (Figure 1c). Overshoot occurs when the gonad elongates past the vulva, extra turn means that the migratory path of DTC includes more than one turn.
To examine the effect of excess NDK-1 exerted on DTC migration, a transgenic strain overexpressing NDK-1 was generated by ballistic transformation. The transgene used for transformation contained a phsp-16.2 NDK-1::mCherry cassette, where NDK-1::mCherry expression is driven by a heat shock promoter. Unfortunately we could not generate an integrated transgenic line, but in our strongest non-integrated line 90% of the animals carried the transgene as extrachromosomal arrays, thus these animals showed NDK-1::mCherry expression in response to heat shock. After heat shock treatment, 14% of mCherry-positive worms (n=42) showed incompletely elongated, J-shaped gonad arms (Figure 1e). The low penetrance of the observed phenotype is likely due to mosaicism of the transgene.
Conclusions
NM23-H1 is Known to Inhibit the Migratory and Invasive Potential of Cancer Cells
Numerous invasive cancer cell lines (eg, breast, colon, oral, hepatocellular carcinoma and melanoma) display low endogenous NM23 levels. In independent experiments using different lines the migratory potential of these cells became significantly lower when transfected with transgenic, exogenous NM23.32, 33, 34, 35, 36, 37, 62 In the opposite scenario, silencing of NM23 in previously non-invasive colon cancer and hepatocellular carcinoma cell lines (expressing higher level of NM23-H1) resulted in an invasive phenotype with altered cell–cell contacts, activation of signal transduction pathways related to tumor progression,38 which are hallmarks of EMT. Some data link the function of NM23-H1 to different members of Rho GTPases, which play an essential role in cell migration and invasion by regulating dynamics of the cytoskeleton. For example NM23-H1 was shown to interact specifically with Tiam1 and Dbl-1, co-factors of Rac1 and Cdc42 respectively, thereby inhibiting the activity of these small GTPases.39, 42
Cystic Fibrosis patients have a higher risk of cancer such as colon cancer with some teenagers even developing metastasis. CDC-42 and the CF protein CFTR have also been linked,63 which is interesting in the context of the binding of both NDPK H1 and H2 in the CFTR hub of proteins, that include potassium channels linked to NDPK function and cell membrane turnover during cell migration has also been reported (cited in Muimo, this series of reviews).
Robust evidence was provided by researchers working on the famous model organism Drosophila that the NM23 homolog AWD functions together with dynamin in endocytosis in a highly specific manner.64 AWD is an inhibitor of cell migration in tracheal morphogenesis and border cell migration.44, 45 In cooperation with Shi/Dynamin, AWD/NDPK suppresses cell motility by downregulating chemotactic receptor levels on the cell surface through receptor internalization.
Our group studied the role of NDK-1, the sole C. elegans group I NDPK homolog in the process of DTC migration. Homozygous ndk-1(−/−) loss-of function mutants showed predominantly incompletely elongated J-shaped gonad arms.58
To better understand the function of NDK-1 in DTC migration, we aimed to fill the missing gaps in our data set: we observed DTC migration patterns in ndk-1+/ndk-1− heterozygotes and generated an ndk-1 overexpressing strain in order to analyze DTC migration phenotypes caused by excess NDK-1. In ndk-1+/ndk-1− heterozygotes, in 24% of the cases ectopically migrating gonad arms (extra turn and overshoot phenotypes) were scored, whereas in nematodes overexpressing NDK-1 again incompletely migrating gonad arms were detected with 14% penetrance (see summarized in Table 1).
The above data suggest that different NDK-1 protein levels lead to diverse DTC migration phenotypes: in the absence of NDK-1 DTC migration is incomplete, wild-type dosage of the ndk-1 gene leads to normal U-shaped gonad arms, half dosage of ndk-1 present in heterozygotes results in extra turns and overshoots of migrating gonad arms, and in response to excess NDK-1 incompletely elongated, J-shaped gonad arms are developed (Table 1).
In Drosophila during tracheal morphogenesis, homozygous awd−/awd− loss-of-function mutants and awd+/awd− heterozygotes show also different phenotypes: in homozygotes a complete disruption of tracheal tubules, whereas in heterozygotes ectopic branch migration was observed,49 Table 1). In our opinion, a parallel can be drawn between DTC migration phenotypes in the nematode and tracheal migration phenotypes in the fruit fly: complete absence of the NDPK homolog results in incomplete migration, but presence of half dosage of NDPK causes ectopic migration in both models (eg, extra gonadal turns in the worm and ectopic tracheal branches in the fly, see Table 1). We note that in cystic fibrosis, where NDPK H1 and H2 are defective in activity and complex formation with other partners, tracheal formation is defective in some babies.65
In Drosophila, overexpression of AWD was studied in the process of border cell migration, where excess AWD resulted in stalled migration of border cells (Table 1). In the nematodes, we overexpressed NDK-1 in the DTCs using a transgenic strain for NDK-1, driven by a heat shock promoter and demonstrated insufficiently elongated gonad arms (Table 1). In both cases, overexpression of NDPK caused an inhibition of cell migration: (1) in the fly border cells did not reach their final position in the egg chamber (eg, they did not arrive to the oocyte), (2) in nematodes the migration of DTCs was blocked at the dorsal phase of gonadal migration where NDK-1 functions.
In Drosophila, the mechanism whereby AWD influences tracheal and border cell migration is well known: AWD works as a local GTP supplier of Shi/Dynamin, which regulates chemotactic receptor levels on the surface of migrating cells (eg, FGFR levels in case of tracheal cells and Pvr levels on border cells) through receptor internalization by its endocytic function.44, 45
In C. elegans, the mechanism whereby NDK-1 influences DTC migration is not yet known, further investigations are needed to better understand its function in this process.
However, DYN-1, the Dynamin worm homolog was shown to function in DTC migration,66 in addition our group identified a genetic interaction between NDK-1 and DYN-1,58 suggesting that an NDK-1/DYN-1-mediated endocytic mechanism might regulate chemotactic receptor levels, which play a role in DTC migration (Figure 2).
NDK-1/DYN-1 might regulate DTC migration through receptor recycling. Distal tip cell (DTC) is a half-moon shaped cell located to the tip of the C. elegans gonad arm. Arrows in bold show the direction of DTC’s movement. A precise level of chemotactic receptors on the surface of DTCs is required for proper pathfinding during certain phases of gonad development. We hypothesize that the process of receptor internalization regulates cell surface receptor levels, such as the level of integrins or netrins in the dorsal phase of migration. NDK-1 genetically interacts with the large GTPase DYN-1/dynamin, and likely fuels it with GTP through its NDPK activity to allow this molecular motor to work efficiently in membrane remodeling events. DYN-1’s membrane fission activity results in release of receptor-containing endocytic vesicles from plasma membranes. These vesicles fuse with early endosomes, which are converted to early sorting endosomes. This sorting process leads either to the fusion with lysosomes (degradation of receptors) or recycling endosomes transport receptors back to the surface of the plasma membrane.
Integrin receptors are known to act in the dorsal/third phase of DTC migration similar to NDK-1.58, 67 The loss-of-function mutant phenotype (J-shaped gonad arms) of ndk-1 is reminiscent of that of pat-3, which encodes a worm beta-integrin.66 Thus, beta-integrin is a good candidate as a receptor present on DTCs, whose cell surface level might be modified by NDK-1/DYN-1 activity.
Taken together, our aim was to examine how the absence, the half dosage of NDK-1 and excess NDK-1 influence the migration of DTCs in C. elegans. We analyzed how different levels of an NDPK affect the migration of the same cell type, a single gonadal leader cell, and found that NDK-1/NDPK has a dosage-dependent function in DTC migration (Table 1). The data suggest that some parallels can be drawn between DTC migration phenotypes in the worm and tracheal and border cell migration phenotypes in the fly, emerging in response to different NDPK protein levels (Table 1). In Drosophila, receptor internalization by Shi/Dynamin-mediated endocytosis is confirmed as a mechanism whereby AWD influences cell migration. In C. elegans, NDK-1’s exact molecular function is not known yet during DTC migration but a DYN-1/NDK-1 mediated endocytosis might have a role in internalization of receptors, such as netrins or integrins, that are known to drive the migration of these gonadal leader cells67 (Figure 2). This issue needs further investigation.
NDPKs function as exclusive GEF-like factors to fuel Dynamin GTPases by GTP to allow them to work with high thermodynamic efficiency in membrane rearrangements.48 In the absence of NDPKs, Dynamin is not fueled efficiently by GTP and decreased Dynamin activity leads to reduced membrane invagination, which might have a consequence on cell surface receptor levels on migrating cells through receptor recycling. To explain how the dosage of NDPKs might influence cell migration, extensive work in Drosophila provides examples. Loss of AWD function was examined in tracheal cells of homozygous and heterozygous awd mutants, and an over accumulation of breathless/FGFR was detected on the surface of tracheal cells.44 Similarly, knockdown of awd in cultured Drosophila S2 cells resulted also in an elevated FGFR level on the cell surfaces.44 Different tracheal phenotypes were observed in case of awd homozygotes and heterozygotes: in the former case a complete disruption of tracheal tubules, whereas in the latter case ectopic branch migration was noted.44, 49 In homozygotes complete loss-of AWD resulted in over accumulation of surface receptor and delayed migration, which is the net outcome of random movement of the cells as a consequence of lack of directional cues due to symmetrical signal activation of the receptor. In heterozygotes the former severe phenotype is rarely seen, in the majority of cases the overall tracheal network is developed often with ectopic branches.
Low level of AWD allows border cells to migrate from the epithelium to the anterior pole of the oocyte during normal oogenesis.45 Overexpression and knockdown of awd was analyzed by the Hsu lab in the process of border cell migration, where both conditions resulted in stalled migration of border cells.45
Ectopic expression and lack of AWD led to similar phenotypes (stalled migration), however influencing the level of Pvr, the chemotactic receptor on border cell surfaces in an opposite way: AWD overexpression resulted in reduced Pvr levels, whereas its knockdown increased the expression of the chemotactic receptor.
Consistently, earlier work have shown that both lack and excess of Pvr signaling impair border cell migration: (1) overexpression of Pvr results in spinning of border cells without moving forward owing to suppressed, nondirectional chemotactic signaling response, (2) pvr loss-of-function border cells do not move, as lack of chemotactic receptors leads to downregulated Pvr signaling.51, 52
We also examined the effect of the worm group I NDPK, NDK-1 in a heterologous system: overexpression of NDK-1 in metastatic MDA-MB231T breast carcinoma cells resulted in inhibition of the migratory potential similar to NM23-H1/H2. Thus, the worm NDPK was able to replace its human counterparts in breast carcinoma cells. However, the mechanism whereby NDK-1 exerts its effect remains to be determined, as in human cell lines H1 and H2 isoforms were shown to act in several different mechanisms, which result in the inhibition of migratory and invasive potential of breast carcinoma cells.
References
Horak CE, Lee JH, Marshall J-C et al. The role of metastasis suppressor genes in metastatic dormancy. APMIS 2008;116:586–601.
Steeg PS, Bevilacqua G, Kopper L et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988;80:200–204.
Hartsough MT, Steeg PS . Nm23/nucleoside diphosphate kinase in human cancers. J Bioenerg Biomembr 2000;32:301–308.
Ilijas M, Pavelic K, Sarcevic B et al. Expression of nm23-h1 gene in squamous-cell carcinoma of the cervix correlates with 5-year survival. Int J Oncol 1994;5:1455–1457.
Ouatas T, Salerno M, Palmieri D et al. Basic and translational advances in cancer metastasis: Nm23. J Bioenerg Biomembr 2003;35:73–79.
Tee Y-T, Chen G-D, Lin L-Y et al. Nm23-H1: a metastasis-associated gene. Taiwan J Obstet Gynecol 2006;45:107–113.
Andolfo I, De Martino D, Liguori L et al. Correlation of NM23-H1 cytoplasmic expression with metastatic stage in human prostate cancer tissue. Naunyn Schmiedebergs Arch Pharmacol 2011;384:489–498.
Harłozińska A, Bar JK, Gerber J . nm23 expression in tissue sections and tumor effusion cells of ovarian neoplasms. Int J Cancer 1996;69:415–419.
Yokoyama A, Okabe-Kado J, Wakimoto N et al. Evaluation by multivariate analysis of the differentiation inhibitory factor nm23 as a prognostic factor in acute myelogenous leukemia and application to other hematologic malignancies. Blood 1998;91:1845–1851.
Agarwal R, Parks R. Nucleoside diphosphokinases. In: Boyer PD (eds). The Enzymes. Academic Press: NY, 1973, pp 307–333.
Desvignes T, Pontarotti P, Fauvel C et al. Nme protein family evolutionary history, a vertebrate perspective. BMC Evol Biol 2009;9:256.
Boissan M, Lacombe M-L . Learning about the functions of NME/NM23: lessons from knockout mice to silencing strategies. Naunyn Schmiedebergs Arch Pharmacol 2011;384:421–431.
Lee M-Y, Jeong W-J, Oh J-W et al. NM23H2 inhibits EGF- and Ras-induced proliferation of NIH3T3 cells by blocking the ERK pathway. Cancer Lett 2009;275:221–226.
Mochizuki T, Bilitou A, Waters CT et al. Xenopus NM23-X4 regulates retinal gliogenesis through interaction with p27Xic1. Neural Dev 2009;4:1.
Marino N, Marshall J-C, Steeg PS . Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1. Naunyn Schmiedebergs Arch Pharmacol 2011;384:351–362.
Postel EH, Berberich SJ, Flint SJ et al. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 1993;261:478–480.
Thakur RK, Kumar P, Halder K et al. Metastases suppressor NM23-H2 interaction with G-quadruplex DNA within c-MYC promoter nuclease hypersensitive element induces c-MYC expression. Nucleic Acids Res 2009;37:172–183.
Fan Z, Beresford PJ, Oh DY et al. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell 2003;112:659–672.
Takács-Vellai K, Vellai T, Farkas Z et al. Nucleoside diphosphate kinases (NDPKs) in animal development. Cell Mol Life Sci 2015;72:1447–1462.
Engel M, Véron M, Theisinger B et al. A novel serine/threonine-specific protein phosphotransferase activity of Nm23/nucleoside-diphosphate kinase. Eur J Biochem 1995;234:200–207.
Klumpp S, Krieglstein J . Reversible phosphorylation of histidine residues in proteins from vertebrates. Sci Signal 2009; 2:pe13.
Ma D, Xing Z, Liu B et al. NM23-H1 and NM23-H2 repress transcriptional activities of nuclease-hypersensitive elements in the platelet-derived growth factor-A promoter. J Biol Chem 2002;277:1560–1567.
Zhang Q, McCorkle JR, Novak M et al. Metastasis suppressor function of NM23-H1 requires its 3’-5’ exonuclease activity. Int J Cancer 2011;128:40–50.
Tokarska-Schlattner M, Boissan M, Munier A et al. The nucleoside diphosphate kinase D (NM23-H4) binds the inner mitochondrial membrane with high affinity to cardiolipin and couples nucleotide transfer with respiration. J Biol Chem 2008;283:26198–26207.
Baughman C, Morin-Leisk J, Lee T . Nucleoside diphosphate kinase B (NDKB) scaffolds endoplasmic reticulum membranes in vitro. Exp Cell Res 2008;314:2702–2714.
Mitchell KAP, Szabo G, de S Otero A . Direct binding of cytosolic NDP kinases to membrane lipids is regulated by nucleotides. Biochim Biophys Acta 2009;1793:469–476.
Epand RF, Schlattner U, Wallimann T et al. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophys J 2007;92:126–137.
Schlattner U, Tokarska-Schlattner M, Ramirez S et al. Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. J Biol Chem 2013;288:111–121.
Steeg PS, Horak CE, Miller KD . Clinical-translational approaches to the Nm23-H1 metastasis suppressor. Clin Cancer Res 2008;14:5006–5012.
Boissan M, Wendum D, Arnaud-Dabernat S et al. Increased lung metastasis in transgenic NM23-Null/SV40 mice with hepatocellular carcinoma. J Natl Cancer Inst 2005;97:836–845.
Leone A, Flatow U, King CR et al. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell 1991;65:25–35.
Tagashira H, Hamazaki K, Tanaka N et al. Reduced metastatic potential and c-myc overexpression of colon adenocarcinoma cells (Colon 26 line) transfected with nm23-R2/rat nucleoside diphosphate kinase alpha isoform. Int J Mol Med 1998;2:65–68.
Liu F, Zhang Y, Zhang X-Y et al. Transfection of the nm23-H1 gene into human hepatocarcinoma cell line inhibits the expression of sialyl Lewis X, alpha1,3 fucosyltransferase VII, and metastatic potential. J Cancer Res Clin Oncol 2002;128:189–196.
Fukuda M, Ishii A, Yasutomo Y et al. Decreased expression of nucleoside diphosphate kinase alpha isoform, an nm23-H2 gene homolog, is associated with metastatic potential of rat mammary-adenocarcinoma cells. Int J cancer 1996;65:531–537.
Baba H, Urano T, Okada K et al. Two isotypes of murine nm23/nucleoside diphosphate kinase, nm23-M1 and nm23-M2, are involved in metastatic suppression of a murine melanoma line. Cancer Res 1995;55:1977–1981.
Parhar RS, Shi Y, Zou M et al. Effects of cytokine-mediated modulation of nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. Int J cancer 1995;60:204–210.
Leone A, Flatow U, VanHoutte K et al. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene 1993;8:2325–2333.
Boissan M, De Wever O, Lizarraga F et al. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res 2010;70:7710–7722.
Otsuki Y, Tanaka M, Yoshii S et al. Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci USA 2001;98:4385–4390.
Horak CE, Lee JH, Elkahloun AG et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res 2007;67:7238–7246.
Van Leeuwen FN, Olivo C, Grivell S et al. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J Biol Chem 2003;278:400–406.
Murakami M, Meneses PI, Knight JS et al. Nm23-H1 modulates the activity of the guanine exchange factor Dbl-1. Int J cancer 2008;123:500–510.
Rosengard AM, Krutzsch HC, Shearn A et al. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature 1989;342:177–180.
Dammai V, Adryan B, Lavenburg KR et al. Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev 2003;17:2812–2824.
Nallamothu G, Woolworth JA, Dammai V et al. Awd, the homolog of metastasis suppressor gene Nm23, regulates Drosophila epithelial cell invasion. Mol Cell Biol 2008;28:1964–1973.
Affolter M, Caussinus E . Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 2008;135:2055–2064.
Schottenfeld J, Song Y, Ghabrial AS . Tube continued: morphogenesis of the Drosophila tracheal system. Curr Opin Cell Biol 2010;22:633–639.
Boissan M, Montagnac G, Shen Q et al. Membrane trafficking. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science 2014;344:1510–1515.
Hsu T . NME genes in epithelial morphogenesis. Naunyn Schmiedebergs Arch Pharmacol 2011;384:363–372.
Montell DJ, Yoon WH, Starz-Gaiano M . Group choreography: mechanisms orchestrating the collective movement of border cells. Nat Rev Mol Cell Biol 2012;13:631–645.
Duchek P, Somogyi K, Jékely G et al. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 2001;107:17–26.
McDonald JA, Pinheiro EM, Montell DJ . PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 2003;130:3469–3478.
Silver DL, Geisbrecht ER, Montell DJ . Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 2005;132:3483–3492.
Dearolf CR, Tripoulas N, Biggs J et al. Molecular consequences of awdb3, a cell-autonomous lethal mutation of Drosophila induced by hybrid dysgenesis. Dev Biol 1988;129:169–178.
Woolworth JA, Nallamothu G, Hsu T . The Drosophila metastasis suppressor gene Nm23 homolog, awd, regulates epithelial integrity during oogenesis. Mol Cell Biol 2009;29:4679–4690.
Bell GP, Thompson BJ . Colorectal cancer progression: lessons from Drosophila? Semin Cell Dev Biol 2014;28:70–77.
Masoudi N, Fancsalszky L, Pourkarimi E et al. The NM23-H1/H2 homolog NDK-1 is required for full activation of Ras signaling in C. elegans. Development 2013;140:3486–3495.
Fancsalszky L, Monostori E, Farkas Z et al. NDK-1, the homolog of NM23-H1/H2 regulates cell migration and apoptotic engulfment in C. elegans. PLoS ONE 2014;9:e92687.
Lehmann R . Cell migration in invertebrates: clues from border and distal tip cells. Curr Opin Genet Dev 2001;11:457–463.
Meighan CM, Schwarzbauer JE . Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev 2007;21:1615–1620.
Annesley SJ, Bago R, Bosnar MH et al. Dictyostelium discoideum nucleoside diphosphate kinase C plays a negative regulatory role in phagocytosis, macropinocytosis and exocytosis. PLoS ONE 2011;6:e26024.
Miyazaki H, Fukuda M, Ishijima Y et al. Overexpression of nm23-H2/NDP kinase B in a human oral squamous cell carcinoma cell line results in reduced metastasis, differentiated phenotype in the metastatic site, and growth factor-independent proliferative activity in culture. Clin Cancer Res 1999;5:4301–4307.
Ferru-Clément R, Fresquet F, Norez C et al. Involvement of the Cdc42 pathway in CFTR post-translational turnover and in its plasma membrane stability in airway epithelial cells. PLoS ONE 2015;10:e0118943.
Krishnan KS, Rikhy R, Rao S et al. Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron 2001;30:197–210.
Diwakar A, Adam RJ, Michalski AS et al. Sonographic evidence of abnormal tracheal cartilage ring structure in cystic fibrosis. Laryngoscope 2015;125:2398–2404.
Cram EJ, Shang H, Schwarzbauer JE . A systematic RNA interference screen reveals a cell migration gene network in C. elegans. J Cell Sci 2006;119:4811–4818.
Wong M-C, Schwarzbauer JE . Gonad morphogenesis and distal tip cell migration in the Caenorhabditis elegans hermaphrodite. Wiley Interdiscip Rev Dev Biol 2012;1:519–531.
Acknowledgements
This work was supported by the OTKA (Hungarian Scientific Research Fund) grant K115587 and MedInProt Protein Science Research Synergy Program (provided by the Hungarian Academy of Sciences) to KTV. AM was funded by grants from the Wellcome Trust and Russell Trust. KT was supported by the KTIA_NAP_13-2014-0018 and VEKOP-2.3.3-15-2016-00007 grants from the National Research, Development and Innovation Office.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
In this review, the authors summarize mechanisms that are linked to the anti-metastatic activity of NM23. Based on data derived from the model organisms C. elegans and Drosophila, they suggest that NDPK enzymes control the availability of surface receptors to regulate cell-sensing cues during cell migration. The dosage of NDPKs may be a coupling factor modulating the efficiency of receptor recycling.
Rights and permissions
About this article
Cite this article
Farkas, Z., Fancsalszky, L., Saskői, É. et al. The dosage-dependent effect exerted by the NM23-H1/H2 homolog NDK-1 on distal tip cell migration in C. elegans. Lab Invest 98, 182–189 (2018). https://doi.org/10.1038/labinvest.2017.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.99
This article is cited by
-
The Function of NM23-H1/NME1 and Its Homologs in Major Processes Linked to Metastasis
Pathology & Oncology Research (2020)
-
NM23 proteins: innocent bystanders or local energy boosters for CFTR?
Laboratory Investigation (2018)
-
The NDPK/NME superfamily: state of the art
Laboratory Investigation (2018)