Abstract
B7-H1 binding to programmed death-1 may deliver a coinhibitory signal to T cells that is involved in the regulation of T-cell activation and tolerance. B7-H1 plays a key role in dysfunction of dendritic cells (DCs) during chronic HBV infection, but the expression mechanism of B7-H1 remains unclear. One hundred and twenty-nine patients with chronic HBV infection were categorized into either the immune tolerance phase (HBV-IT), the immune clearance phase (HBV-IC), or the inactive carrier phase (HBV-IA). Twenty healthy volunteers were enrolled as controls. Another 16 patients with HBeAg-positive chronic Hepatitis B were enrolled, and entecavir was administrated at 0.5 mg per day for 6 months. The B7-H1 expression level on peripheral DCs was tested by flow cytometry. In vitro, expression levels of B7-H1 and signaling molecules on monocyte-derived DC (MO-DC) induced by recombinant hepatitis B virus C antigen (rhHBcAg) were examined by RT-PCR, flow cytometry, and western blotting, and the apoptosis rate was tested by flow cytometry. The percentages of peripheral DCs and myeloid DCs (mDCs) were decreased and B7-H1 levels were increased in patients compared with controls. Serum HBV-DNA levels were positively correlated with B7-H1 levels on mDCs in patients with HBV-IT. B7-H1 levels on peripheral DCs from patients with chronic hepatitis B decreased after antiviral therapy. In vitro studies demonstrated that the B7-H1 level on MO-DC was upregulated by rhHBcAg, which resulted from the activation of PI3K–AKT, ERK, and P38 signaling pathways, and the percentage of MO-DC was downregulated by rhHBcAg. In addition, rhHBcAg promoted the apoptosis of MO-DC. The data suggest that HBcAg induced B7-H1 upregulation by activating AKT, ERK, and P38 signaling pathways, which inhibited the clearance of HBV-DNA and the reduction of DCs contributed to immune tolerance, which may correlate with apoptosis.
Similar content being viewed by others
Main
Chronic hepatitis B virus (HBV) infection is an important public health problem worldwide and highly correlates with the occurrence of hepatocirrhosis and hepatic carcinoma.1, 2, 3 The outcome of HBV infection and pathogenesis of liver disease are immune mediated and thus determined by the virus–host interaction.4 Chronic infection may persist for life and cause varying grades of chronic liver injury, which may lead to hepatocirrhosis and/or hepatocellular carcinoma.5 The immune stages of chronic HBV infection is clinically categorized into three periods: the immune tolerance phase (HBV-IT), immune clearance phase (HBV-IC), and immune stable phase or inactive virus carrier phase (HBV-IA).6, 7, 8
Dendritic cells (DCs) are the most potent antigen-presenting cell type, with the unique ability to induce not only primary immune responses against invading pathogens but also immunological tolerance.9 Two major DC populations can be identified in peripheral blood: CD11c+ myeloid DCs (mDCs) and CD123+ plasmacytoid DCs (pDCs).10 DCs are key players in antigen presentation and initiation of virus-specific T-cell responses, and it has been reported that mDCs and pDCs are functionally impaired in patients with chronic hepatitis B (CHB).11 It has been determined that a decrease of HBV-DNA levels may result in the improvement of mDC function.12 Another team found that HBV particles and purified HBV surface antigen had an immune modulatory capacity and may directly contribute to the dysfunction of mDCs in patients with chronic HBV.13
B7-H1 (also called CD274 or programmed death ligand 1) is a cell surface glycoprotein belonging to the B7 family of costimulatory molecules.14 B7-H1 binding to programmed death-1 (PD-1) could deliver a coinhibitory signal to T cells, which is involved in the regulation of T-cell activation and tolerance.15, 16, 17, 18 Wang et al19 found that the B7-H1 upregulation on mDCs significantly suppressed T-cell immune function in CHB patients. Many other studies showed that targeting the PD-1/B7-H1pathway can mobilize the immune system, which may indicate an innovative potential therapy for cancers and chronic infections.20 Additionally, it has been shown that hepatitis B envelope antigen (HBeAg) is able to significantly upregulate B7-H1 expression.21
Our previous study22 showed that the PD-1 expression level on CD4+T cells was upregulated during chronic HBV infection, which was induced by recombinant hepatitis B virus C antigen (rhHBcAg) through JNK, ERK, and PI3K/AKT signaling pathways. However, the expression levels of B7-H1 during chronic HBV infection and the relevant mechanisms were not elucidated; such information could be very important for inhibiting the PD-1/B7-H1 pathways. In the present paper, our data show that the reduction of the frequency of DCs contributed to immune tolerance and the upregulation of B7-H1 expression on DCs inhibited the clearance of HBV-DNA in patients with chronic HBV infection, which correlated with the clinical efficacy of entecavir. In vitro, the frequency of monocyte-derived DC (Mo-DC) was decreased and the B7-H1 level on Mo-DC was upregulated by rhHBcAg through activating AKT, ERK, and P38 signaling pathways, which may be correlated with the apoptosis of Mo-DC.
MATERIALS AND METHODS
Patients and Healthy Controls
One hundred and twenty-nine patients with chronic HBV infection were enrolled (Supplementary Table S1), who were positive for HBV surface antigen and anti-HBc, but negative for antibodies to HCV, hepatitis-D virus, HIV-1, and HIV-2, and had no other symptoms of chronic liver damage. No patients were treated for chronic HBV infection or received any other medication 72 weeks prior to blood sampling. Among the129 patients, 53 were in the HBV-IT phase, 57 were in the HBV-IC phase, and 19 were in the HBV-IA phase. The healthy control (HC) group, matched for age, sex, and race, comprised 20 healthy volunteers with no evidence of liver diseases and negative for HBV and HCV. Another 16 HBeAg-positive CHB patients (Supplementary Table S2) were enrolled, and they were treated with entecavir 0.5 mg daily for 6 months. All patients and healthy controls were Chinese. Our study was approved by the local ethics committee and all patients provided written informed consent according to the protocol reviewed and approved by the institutional review board of Shanghai Shuguang Hospital.
Serum Viral Load, Alanine Transaminase (ALT), and Aspartate Transaminase (AST) Determination
Serum HBV-DNA levels in patients with chronic HBV infection were quantified by the Light Cycler PCR system (FQD-33A). The lower limit of detection of this assay was 1000 viral genome copies per milliliter.
Serum ALT and AST levels were assayed by using the DXC 800 Fully-auto Bio-Chemistry Analyzer. The results were considered abnormal when ALT was >60 U/l and/ or AST was >60 U/l.
All detections were completed at the Department of Clinical Laboratory, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, China.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from the heparinized blood by standard density-gradient centrifugation using Lympholyte-H (Cedarlane) according to the manufacturer’s protocol.
Mo-DC Preparation
Peripheral monocytes were isolated from PBMCs of healthy volunteers using Dynabeads Untouched Human monocytes kit (Invitrogen Dynal As, Oslo, Norway) according to the manufacturer’s protocol. To generate immature Mo-DC,23, 24 the isolated monocytes were cultured at a cell density of 5 × 105 cells/ml in culture medium, in the presence of recombinant IL-4 (20 ng/ml) and recombinant GM-CSF (50 ng/ml) for 6–7 days. Every 3 days, the half of the medium was removed and was exchanged with the new medium containing the same cytokines. To generate mature Mo-DC, immature Mo-DC was cultured in the presence of TNF-α (20 ng/ml) for another 3 days. Mo-DC was identified by flow cytometry (flow cytometry) (Figure 4a).
Real-Time RT-PCR
RNA was treated with RNase-free DNase-I (Takara Bio) and reverse-transcribed using avian myeloblastosis virus reverse transcriptase (Promega). Real-time PCR was performed using the Mastercycler ep realplex 4 real-time PCR system (Eppendorf) with an SYBR Green qPCR Master Mix (Fermentas), according to the manufacturer’s protocol. The sequences of primers for human B7-H1 (NM_ 014143.3) were 5′-TGCGTTCAGCAAATGCCAGT-3′ (forward) and 5′- ATTGCAGGATGCAGGGGTGTA-3′ (reverse). The primers for human GAPDH (NM_002046.3) were 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse). The amplification conditions were as follows: 95 °C for 10 min, and 40 cycles of 95 °C for 15 s, 63 °C for 30 s, and 72 °C 20 s. The amount of B7-H1 transcripts of individual samples was normalized to GAPDH.
Flow Cytometry
PBMCs were stained with Peridinin–Chlorophyll Protein Complex (PerCP)-, FITC-, phycoerythrin (PE)-, and allophycocyanin (APC)-labeled mAbs, respectively, according to the instructions of the respective manufacturers. Flow cytometry was performed using FACSAria (Becton Dickinson, San Jose, CA, USA). FITC-anti-lin 1, PerCP-anti-HLA-DR, APC-anti-CD11c, and PE-anti-B7-H1 mAbs, and isotype-matched control antibodies were purchased from BD Biosciences (San Jose, CA, USA).
Apoptosis of Mo-DC was examined by flow cytometry. Mo-DC was stained by FITC-annexin V, propidium iodide (PI), (BD Biosciences). After staining, cells were washed twice and resuspended in phosphate-buffered saline, and flow cytometry was performed using FACSAria (Becton Dickinson). Early apoptotic cells were identified as annexin V+ PI- events; late apoptotic cells were identified as annexin V+ PI+events.
Gene Expression After In Vitro Stimulation and Inhibitor Experiment
RhHBcAg (10 μg/ml, HBV-232, Prospec-Tany Techno Gene) was added to the culture medium of Mo-DC to examine its effects on B7-H1 expression in every group. Mo-DC was respectively cultured for 0, 12, 24, 48, and 72 h in 24-well cell culture plates at 37 °C under 5% CO2. Then Mo-DC was collected and B7-H1 expression was examined by real-time RT-PCR, flow cytometry, and western blotting. In the inhibitor experiment, Mo-DC was incubated with LY294002 (PI3K/AKT inhibitor, 25 mM), U0126 (ERK inhibitor, 150 nM), SB203580 (p38 inhibitor, 1 mM), and SP600125 (JNK inhibitor, 100 nM) for 1 h and then rhHBcAg (10 mg/ml) was added to the culture medium of every group. After 48 h, Mo-DC was collected and B7-H1 expression was examined by flow cytometry and western blotting. LY294002 (V1201), U0126 (V1121) and SB203580 (V1161) were purchased from Promega Corporation. SP600125 (s5567) was purchased from Sigma.
Western Blottings
Cells were washed twice with ice-cold PBS and prepared with RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate and 0.1% SDS) containing protease inhibitor mixture (Roche). The samples were separated by SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane (Millipore) using SemiDry Transfer Cell (Bio-Rad). The polyvinylidene difluoride membrane was blocked with 5% non-fat milk and incubated with the first antibodies at 4 °C overnight. The blots were incubated with the HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. The antibodies against AKT/p-AKT, ERK/p-ERK, JNK/p-JNK, and p38/p-p38 were purchased from Santa Cruz Biotechnology.
Statistical Analysis
One-way ANOVA was used to compare multiple groups and two groups using the SPSS software. Pearson correlation analyses of B7-H1 expression and HBV DNA copies, and the ALT level, were performed. Differences were considered statistically significant at P<0.05. The test of significance was two sided.
RESULTS
Upregulation of B7-H1 Expression on Peripheral DCs in Patients with Chronic HBV Infection
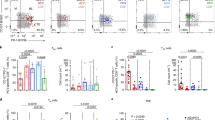
DCs (lin1-/HLA-DR+) and mDCs (lin1-/HLA-DR+/CD11c+) were characterized according to the cell surface markers, as shown in Supplementary Figure S1. The characteristics of DCs and mDCs in PBMCs isolated from 129 patients with chronic HBV infection and 20 HCs were compared. The percentages of DCs and mDCs were decreased in patients compared with that in HCs, and it was gradually increased from HBV-IT, HBV-IC to HBV-IA (Figure 1a–d), and the expression levels of B7-H1 on DCs and mDCs were significantly increased in all patients, as shown in Figure 1e–h.
Upregulation of B7-H1 expression on peripheral DCs in patients with chronic HBV infection. (a, b) The dot plots showing the percentage of DCs in patients with chronic HBV infection and HCs and the horizontal bars indicating the median percentage of DCs. (c, d) The dot plots showing the percentage of mDCs in patients with chronic HBV infection and HCs and the horizontal bars indicating the median percentage of mDCs. (e, f) The dot plots showing the MFI of B7-H1 on DCs in patients with chronic HBV infection and HCs and the horizontal bars indicating the median MFI of B7-H1 on DCs. (g, h) The dot plots showing the MFI of B7-H1 on mDCs in patients with chronic HBV infection and HCs and the horizontal bars indicating the median MFI of B7-H1 on mDCs. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Relationships between B7-H1 Expression Level on Peripheral DCs and Serum ALT Levels and HBV-DNA Levels
The results showed that there were no significant correlations between B7-H1 expression level on DCs or mDCs and serum ALT levels in all patients (Supplementary Figure S2), and there were no significant correlations between B7-H1 expression level on DCs and serum ALT levels even in every immune phase (Supplementary Figure S3). It was interesting that the B7-H1 expression level in mDCs was positively correlated with serum ALT level in HBV-IT phase (Figure 2a). In addition, the serum HBV-DNA level was positively correlated with B7-H1 on mDCs in patients (Supplementary Figure S4). Especially, in HBV-IT patients, B7-H1 expression level on mDCs was positively correlated with serum HBV-DNA level (Figure 2b), which showed that the upregulation of B7-H1 on mDCs inhibited the clearance of serum HBV DNA. Interestingly, in HBV-IA patients, serum HBV-DNA level was negatively correlated with B7-H1 expression level on DCs (Supplementary Figure S5), which suggested that although HBV DNA was cleared, the upregulation of B7-H1 on DCs was persistent.
Antiviral Treatment Reduced the B7-H1 Expression on Peripheral DCs in CHB Patients
In order to explore the role of B7-H1 expression on peripheral DCs against chronic HBV infection, 16 patients with HBeAg-positive CHB were treated with antivirus drug (0.5 mg entecavir daily) for 6 months. The antiviral treatment effectively decreased serum ALT/AST levels and HBV-DNA load (Figure 3a and b). Importantly, the percentage of DCs was markedly increased, and the MFI of B7-H1 on DCs was significantly decreased after antiviral therapy (Figure 3c and d).
B7-H1 expression levels on peripheral DCs in CHB patients after antivirus therapy. (a) Serum ALT and AST levels of patients before and after entecavir treatment. (b) Serum HBV-DNA levels before and after entecavir treatment. (c) The percentages of peripheral DCs before and after entecavir treatment. (d) The MFI of B7-H1 on peripheral DCs before and after entecavir treatment. *P<0.05; ***P<0.001.
The Percentage of mDCs was Decreased by rhHBcAg In Vitro Experiments
In this experiment, peripheral monocytes were isolated and were induced to Mo-DC, and then Mo-DC was identified by flow cytometry. Results from flow cytometry showed that most of Mo-DC expressed the same markers as mDCs (lin1−/HLA-DR+/CD11c+). Then rhHBcAg (10 μg/ml) was added into the culture medium of Mo-DC at different time points. Results showed that the percentage of DCs was increased at 24, 48, and 72 h and the B7-H1 level was upregulated at all time points after incubation of rhHBcAg (Figure 4a–c). However, the percentages of mDCs were decreased at all time points and B7-H1 levels were upregulated at all time points after incubation of rhHBcAg (Figure 4d–f), which was similar with the result in patients with HBV-IT (Figure 1). Therefore, HBcAg contributes to the decrease of mDCs during chronic HBV infection, which may be correlated with the upregulation of B7-H1 on mDCs.
The percentages of mDCs were decreased by rhHBcAg. Mo-DC from peripheral blood of healthy people was incubated with rhHBcAg for indicated times. (a) The dot plots showing the percentage of DCs when Mo-DC was incubated with rhHBcAg for indicated times. (b) The percentage of DCs was examined by flow cytometry. (c) The MFI of B7-H1 on DCs was examined by flow cytometry. (d) The dot plots showing the percentage of mDCs when Mo-DC was incubated with rhHBcAg for indicated times. (e) The percentage of mDCs was examined by flow cytometry. (f) The MFI of B7-H1 on mDCs was examined by flow cytometry. *P<0.05; **P<0.01; ***P<0.001.
Upregulation of B7-H1 Expression on Mo-DC Induced by rhHBcAg through AKT, JNK, and P38 Signaling Pathway
RhHBcAg (10 μg/ml) was added into the culture of Mo-DC at different time points, and then the upregulation of B7-H1 expression induced by rhHBcAg was shown in Figure 6a. Next, p38, AKT, ERK, and JNK pathways on Mo-DC were individually inhibited by using the relevant inhibitors. Results from flow cytometry and western blotting showed that the inhibition of p38, AKT, and ERK pathways reduced the upregulation of B7-H1 induced by rhHBcAg (Figure 5b). In addition, p38, AKT, and ERK pathways were activated by rhHBcAg (Figure 5c). The above results show that the expression level of B7-H1 on Mo-DC is upregulated by rhHBcAg through activation of p38, AKT, and ERK pathways.
Upregulation of B7-H1 expression on DCs induced by rhHBcAg through the AKT, JNK, and P38 signaling pathway. (a) Mo-DC was incubated with rhHBcAg for different times, and B7-H1 expression levels were examined by RT-PCR, flow cytometry and western blotting. (b) DCs were pretreated with U0126 (150 nM), SP600125 (100 nM), LY294002 (25 μM), and SB203580(1 μM) for 1 h, and then were incubated with rhHBcAg for 48 h. The normal DCs were used as a negative control. B7-H1 expression levels were examined by flow cytometry and western blotting. (c) DCs were incubated with rhHBcAg for indicated times, and the phosphorylated forms of P38, AKT, ERK, and JNK were detected by western blotting using phosphorylation-specific Abs. The blots were re-probed for total P38, AKT, ERK, and JNK. *P<0.05; **P<0.01.
The Apoptosis of DCs was Induced by rhHBcAg
In the above results, we found that the percentages of peripheral DCs in patients with chronic HBV infection were decreased and in vitro the upregulation of B7-H1 was induced by rhHBcAg. Seong Jeong Park et al25 showed that PD-1/B7-H1 pathway played an important role in apoptosis of activated DCs. So we wanted to explore whether the reduction of Mo-DC induced by rhHBcAg was correlated with the apoptosis. The frequency of apoptotic Mo-DC induced by rhHBcAg was examined by flow cytometry, and the results showed that both the early apoptotic DCs (Annexin V+/PI−) and late apoptotic DCs (Annexin V+/PI+) were increased after incubation with rhHBcAg (Figure 6).
The apoptosis of DCs was induced by rhHBcAg. (a) Representative dot plots illustrating the early apoptotic DCs (Annexin V+) and late apoptotic DCs (Annexin V+PI+) after incubation with rhHBcAg. (b) The analytic data of the early apoptotic DCs after incubation with rhHBcAg. (c) The analytic data of the late apoptotic DCs after incubation with rhHBcAg. *P<0.05; **P<0.01; ***P<0.001.
DISCUSSION
Chronic HBV infection is the result of an inadequate immune response towards the virus. DCs play an important role in antiviral immunity and have the unique capacity to activate naïve T cells and stimulate B cells and natural killer cells.26, 27 In patients with chronic HBV infection, the maturation and function of mDCs are impaired,11, 28 resulting in more tolerogenic rather than immunogenic responses, which may contribute to viral persistence. The mechanism responsible for the altered function of mDCs remains unclear. Our results show that the percentage of peripheral mDCs is reduced and the B7-H1 expression is increased in every phase of patients with chronic HBV infection, and in vitro experiments with rhHBcAg show that the percentage of Mo-DC (lin1−/HLA-DR+/CD11c+) that expresses the same markers as mDC is decreased and its B7-H1 expression is increased, which suggests that the decrease of mDCs may be correlated with the upregulation of B7-H1.
B7-H1 is expressed by most cell types including DCs, while its receptor PD-1 is only present on certain immune cells, such as activated T cells and regulatory T cells.29, 30, 31 Under physiological conditions, B7-H1 binding to PD-1 is critical in the maintenance of peripheral T-cell tolerance, preventing autoimmune responses.32 In this paper, the correlation between the serum HBV-DNA level and the B7-H1 level on mDCs in patients with HBV-IT phase is positive, which suggests that the upregulation of B7-H1 on mDCs is invloved in the persistence of HBV. It is interesting that in the HBV-IT phase, the correlation between serum ALT levels and the B7-H1 level on mDCs is positive, which provides us a new information that the B7-H1 level on mDCs in these patients should be closely observed. Modification of human Mo-DC with the soluble extracellular part of PD-1 or B7-H1 mRNA results in increased levels of the costimulatory molecule CD80, IL-10, and TNF-α,33 which suggests that the blockade of the PD-1/B7-H1 pathway is one of DC-based immunotherapeutic strategies. Chen et al34 found that the labeling indices of PD-1 and B7-H1 in lymphocytes infiltrating portal area were significantly higher in CHB patients than in healthy controls, the increases in labeling indexes of PD-1 and B7-H1 paralleled the degree of inflammation, but the B7-H1 level on DCs was not examined. Our present studies show that B7-H1 expression level in peripheral DCs is upregulated in patients with chronic HBV infection, especially in the HBV-IT phase, and in addition, the results from in vitro experiments show that the upregulation of B7-H1 expression on Mo-DC is induced by rhHBcAg through activating PI3K/AKT, ERK, and P38 signaling pathways. In addition, the B7-H1 expression level in peripheral DCs is decreased and the percentage of DCs is increased after antiviral treatment in patients with chroinc hepatitis B. However, the data in three patients deserve our attention, and their peripheral percentages of DCs are not increased and the B7-H1 expression level in one patient is decreased and the B7-H1 expression levels in another two patients are not decreased. Seong Jeong Park et al25 showed that the PD-1/B7-H1 pathway played an important role in apoptosis of activated DCs. So maybe the decrease of B7-H1 is shown at first, and then the increase of the percentage of DCs will be shown in the longer course of treatment, which will be explored in our future studies. In our present studies, the results show that the apoptotic DCs are increased after incubation with rhHBcAg, which may be correlated with the upregulation of B7-H1. Tzeng et al35 found that PD-1 blockage reversed immune dysfunction and viral persistence of HBV infection in a mouse animal model, suggesting that the blockage of PD-1/B7-H1 pathway might be a good therapeutic candidate for chronic HBV infection. In summary, B7-H1 expression on peripheral DCs is upregulated in patients with HBV-IT, which inhibits the clearance of HBV DNA, and the activation of PI3K/AKT and ERK signaling pathways is correlated with the upregulation of PD-1/B7-H1 induced by rhHBcAg in vitro, which provides the potential therapeutic methods for blocking the PD-1/B7-H1 pathways by inhibiting the activation of PI3K/AKT and ERK signaling pathways.
References
Lai CL, Yuen MF . Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology 2013;57:399–408.
Papatheodoridis GV, Chan HL, Hansen BE et al. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 2016;62:956–967.
Ringelhan M, O'Connor T, Protzer U et al. The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets. J Pathol 2016;235:355–367.
Bertoletti A, Gehring A . Immune response and tolerance during chronic hepatitis B virus infection. Hepatol Res 2007;37 (Suppl 3):S331–S338.
Webster GJ, Bertoletti A . Control or persistence of hepatitis B virus: the critical role of initial host-virus interactions. Immunol Cell Biol 2002;80:101–105.
Lok AS, McMahon BJ . Chronic hepatitis B. Hepatology 2007;45:507–539.
Pan CQ, Zhang JX . Natural history and clinical consequences of hepatitis B virus infection. Int J Med Sci 2005;2:36–40.
Shi YH, Shi CH . Molecular characteristics and stages of chronic hepatitis B virus infection. World J Gastroenterol 2009;15:3099–3105.
Foti M, Granucci F, Pelizzola M et al. Dendritic cells in pathogen recognition and induction of immune responses: a functional genomics approach. J Leukoc Biol 2006;79:913–916.
Steinman RM . The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991;9:271–296.
van der Molen RG, Sprengers D, Binda RS et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004;40:738–746.
Pan X, Yao W, Fu J et al. Telbivudine improves the function of myeloid dendritic cells in patients with chronic hepatitis B. Acta Virol 2012;56:31–38.
Op den Brouw ML, Binda RS, van Roosmalen MH et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 2009;126:280–289.
Chen L . Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4:336–347.
Dong H, Zhu G, Tamada K et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–1369.
Martin-Orozco N, Wang YH, Yagita H et al. Cutting edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol 2006;177:8291–8295.
Kuipers H, Muskens F, Willart M et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol 2006;36:2472–2482.
Brown JA, Dorfman DM, Ma FR et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003;170:1257–1266.
Chen L, Zhang Z, Chen W et al. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol 2007;178:6634–6641.
Abdel-Magid AF . Inhibitors of the PD-1/PD-L1 pathway can mobilize the immune system: an innovative potential therapy for cancer and chronic infections. ACS Med Chem Lett 2015;6:489–490.
Han Y, Li J, Jiang L et al. Regulation of B7-H1 expression on peripheral monocytes and IFN-gamma secretion in T lymphocytes by HBeAg. Cell Immunol 2013;283:25–30.
Li M, Sun XH, Zhu XJ et al. HBcAg induces PD-1 upregulation on CD4+T cells through activation of JNK, ERK and PI3K/AKT pathways in chronic hepatitis-B-infected patients. Lab Invest 2012;92:295–304.
Sallusto F, Lanzavecchia A . Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179:1109–1118.
Ardavin C, Martinez del Hoyo G, Martin P et al. Origin and differentiation of dendritic cells. Trends Immunol 2001;22:691–700.
Park SJ, Namkoong H, Doh J et al. Negative role of inducible PD-1 on survival of activated dendritic cells. J Leukoc Biol 2014;95:621–629.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998;392:245–252.
Moretta A . Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol 2002;2:957–964.
Duan XZ, Zhuang H, Wang M et al. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J Gastroenterol Hepatol 2005;20:234–242.
Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704.
Punkosdy GA, Blain M, Glass DD et al. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci USA 2011;108:3677–3682.
Wang W, Lau R, Yu D et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009;21:1065–1077.
Dong H, Strome SE, Salomao DR et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800.
Pen JJ, Keersmaecker BD, Heirman C et al. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther 2014;21:262–271.
Chen J, Wang XM, Wu XJ et al. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm Res 2011;60:47–53.
Tzeng HT, Tsai HF, Liao HJ et al. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS One 2012;7:e39179.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81202662, 81473477, 81403354, 81473629, 81403351 and 81503545) and Science Research Project of Twelve Five-year Plan (2012ZX10005004-002) and Three-year action plan of development of TCM in Shanghai (ZYSNXD-CC-ZDYJ015 and ZY3-CCCX-3-3027) and Training plan of outstanding young medical talents, Shanghai Municipal Health Bureau (XYQ2013093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, M., Zhou, ZH., Sun, XH. et al. Hepatitis B core antigen upregulates B7-H1 on dendritic cells by activating the AKT/ERK/P38 pathway: a possible mechanism of hepatitis B virus persistence. Lab Invest 96, 1156–1164 (2016). https://doi.org/10.1038/labinvest.2016.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.96
This article is cited by
-
Integrating pharmacokinetics and network analysis to investigate the mechanism of Moutan Cortex in blood-heat and blood stasis syndrome
Chinese Medicine (2022)
-
Cholesterol accumulation on dendritic cells reverses chronic hepatitis B virus infection-induced dysfunction
Cellular & Molecular Immunology (2022)