Abstract
Interleukin (IL)-33 is a novel IL-1 family member, and its administration has been associated with promotion of T helper type-2 (Th2) cell activity and cytokines, particularly IL-4 and IL-5 in vivo. Recently, IL-33 was shown to increase CD4+Foxp3+ regulatory T cells (Tregs) and to suppress levels of the Th1-type cytokine IFN-γ in allogeneic heart transplantation in mice. Therefore, we hypothesized that IL-33 and leflunomide (Lef) could prolong graft survival in the concordant mouse-to-rat heart transplantation model. In this model, xenografts undergo acute humoral xenograft rejection (AHXR) typically on day 3 or cell-mediated rejection approximately on day 7 if AHXR is inhibited by Lef treatment. Recipients were treated with Lef (n=6), IL-33 (n=6), IL-33 combined with Lef (n=6), or left untreated (n=6) for survival studies. Heart grafts were monitored until they stopped beating. Mouse heterotopic grafts were performed, and recipients were sacrificed on days 2 and 7 for histological and flow cytometric analyses. The combination of IL-33 and Lef significantly prolonged the grafts from 17.3±2.3 to 2.8±0.4 days, compared to untreated controls. IL-33 administration with Lef, while facilitating Th2-associated cytokines (IL-4 on day 2 but not day 7), also decreased IFN-γ on day 2 and day 7, compared with Lef treatment only. Furthermore, IL-33 with Lef administration caused an expansion of suppressive CD4+Foxp3+ Tregs in rats. The IL-33 and Lef combination therapy resulted in significantly prolonged graft survival, associated with markedly decreased Th1 cells and increased IL-10 levels. In addition, the combination therapy significantly decreased the percentage of CD-45+ B cells on days 2 and 7, compared with monotherapy. These findings reveal a new immunoregulatory property of IL-33. Specifically, it facilitates regulatory cells, particularly functional CD4+Foxp3+ Tregs that underlie IL-33-mediated cardiac xenograft survival. Moreover, it can decrease Th1 cells and cytokine expression of Th1 T cells in xenograft recipients, for example IFN-γ.
Similar content being viewed by others
Main
Acute vascular rejection (AVR) or delayed xenograft rejection (DXR) is now generally considered to be the greatest immune challenge for xenotransplantation.1 In concordant models, such as the mouse-to-rat heart transplantation model, the organs undergo cell-mediated rejection when the acute humoral xenograft rejection (AHXR), also known as DXR, is suppressed by sustained treatment with a B-lymphocyte inhibitor.2 AHXR occurs within 3 days after transplantation in the mouse-to-rat and hamster-to-rat heart transplantation models, characterized by binding of different immunoglobulins (Ig) to the vascular endothelium, vasculitis, thrombosis, tissue destruction, and edema.3 Turnquist et a l4 observed a potent ability of interleukin (IL)-33 to increase suppressive CD4+Foxp3+ regulatory T cells (Tregs). Overall, fewer infiltrated T cells, but increased numbers of Tregs, were seen in the heart grafts.4 Furthermore, Tregs were shown to be crucial for the function of IL-33 in promoting cardiac allograft survival, as depletion of these cells before transplantation counteracted any therapeutic effect of the IL-33 treatment.5 Tregs are now appreciated to acquire tissue-specific adaptations that support their survival and function.6
Leflunomide (Lef) is a novel immunosuppressive agent that can inhibit B-cell proliferation and differentiation by interfering with pyrimidine metabolism and by blocking some tyrosine kinases. It may also suppress nuclear factor-B activation and gene expression stimulated by tumor necrosis factor (TNF) and inhibit endothelial functions related to angiogenesis.7 Hassikou et al8 reported that the main adverse effects of Lef include gastrointestinal symptoms, liver enzyme elevation, hepatotoxicity, hypertension, and urinary infections. The toxic effects of Lef, especially at higher concentrations, are solely due to an active metabolite, teriflunomide (formerly A77 1726).9 Therefore, the use of Lef in small doses to achieve prolonged graft survival is particularly essential in order to minimize its toxicity.
IL-33 is a novel member of the IL-1 family, which includes IL-1β and IL-18.10 However, unlike IL-1β and IL-18, which mainly promote Th1-associated responses, IL-33 generally stimulates production of Th2 cytokines (IL-4 and IL-5), and increases serum Ig levels.4 However, new studies indicate that IL-33 is a multifunctional protein that acts as transcriptional/signaling repressor, functions as an alarming alerting the immune system to necrosis, as well as serves as a cytokine that targets cells expressing ST2, the IL-33 receptor.5 Interestingly, functional analysis of IL-33-deficient mice has suggested that IL-33 amplifies both Th1 and Th2 responses, in particular by affecting innate immune cells in the mucosa. Moreover, IL-33 can stimulate production of inflammatory cytokines, such as TNF-α, IL-1b, and IL-6, from mouse macrophages.11 Recently, IL-33 was reported to be able to suppress mRNA levels of IFN-γ, a Th1-type cytokine, in both the allograft and recipient spleen via induction of a Th1-to-Th2 switch in animal models of vascularized allogeneic transplantation.12 In addition, IL-33 treatment has been found to expand suppressive CD4+Foxp3+ Tregs while facilitating Th2 responses in allogeneic cardiac-transplanted mice.4
We hypothesized that IL-33 potentially could be used to decrease leukocyte infiltration and expand CD4+Foxp3+ Tregs during cell-mediated xenograft rejection. To confirm the hypothesis, we used the concordant mouse-to-rat transplantation model, where the donor heart is heterotopically transplanted to the neck vessels of the recipient.3 The combined use of IL-33 and Lef thus allowed us to study the impact of IL-33 on AVR.13 Graft survival was determined in separate animals, and the activation and infiltration of immune cells were studied separately by flow cytometry. Furthermore, we report the novel observation that administering the combination of IL-33 and Lef to cardiac-transplanted rats also expanded suppressive CD4+Foxp3+ Tregs while reducing Th1 responses and IFN-γ levels.
MATERIALS AND METHODS
Animals
Female C57BL/6 (B6, H-2b) mice and Lewis rats (8–12-weeks-old) were purchased from Slac Laboratory Animal (Shanghai, China). All animals were housed in the specific pathogen-free facility of the University of Xiamen at 22 °C with 50% air humidity and air change 45 times per hour. Animals had unlimited access to food and water. Mice weighing 20–25 g were used as donors, and rats weighing 180–220 g were used as recipients. All procedures were performed according to guidelines of the Institutional Animal Care and Use Committee (IACUC).
Heterotopic Heart Transplantation Model
Hearts from B6 mice were transplanted to Lewis rat recipients heterotopically using a non-suture cuff technique. Briefly, the ascending aorta of the graft was concatenated with the right common carotid artery of the recipient, and the pulmonary artery of the donor was connected to the right internal jugular vein of the recipient. Plastic tubes were used to surround the vessels, which were everted over the tube and fixed with 6/0 silk ligatures. The ascending aorta of the donor heart was pulled over the carotid tube of the recipient and the pulmonary artery over the jugular tube, each fixed with a 6/0 silk ligature. Graft survival was monitored daily by palpation. Rejection was defined as the complete loss of a palpable heart beat.
Expression and Purification of Recombinant IL-33
Expression and purification of recombinant IL-33-glutatione S-transferase (GST) were carried out as previously described.12 The purity of the recombinant IL-33 was >95%.
Drug Treatment
For survival studies, recipients were separated into four treatment groups: control (untreated), Lef-only (5 mg/kg body weight) from day −1 until the xenografts stopped beating; IL-33-only (30 μg daily) from day 0 until the xenografts stopped beating; combination of Lef (5 mg/kg body weight) and IL-33 (30 μg daily). IL-33 was injected intraperitoneally until grafts were rejected. Lef was given daily at a dose of 5 mg/kg body weight.
Histological Analysis
The heart xenografts were resected from the recipient rat on days 2 and 7 post transplantation. Biopsy samples of grafts were histologically analyzed using light microscopy. The specimens were fixed with 10% phosphate-buffered formaldehyde for at least 1 day and subsequently dehydrated, paraffin embedded, and cut into 5-μm-thick sections. After staining with hematoxylin and eosin, the sections were evaluated in a blinded fashion. Graft rejection was graded on the extent of infiltration and the anatomical localization of inflammatory cells according to the International Society of Heart and Lung Transplantation (ISHLT) standard.14
Mixed Lymphocyte Reactions (MLR)
T lymphocytes isolated from recipient rat spleens using nylon wool columns (Wako, Osaka, Japan) were used as responder cells, and spleen cells of donor B6 mice were used as stimulator cells. The responder cells (5 × 105 cells) were cultured with the stimulator cells (5 × 104 cells) pre-treated with mitomycin C (40 μg/ml, Amresco, Solon, OH, USA) in 200 μl RPMI1640 supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin. Each reaction was carried out in triplicate. The cells were then cultured at 37 °C in a humidified atmosphere with 5% CO2 for 3 days.15 Subsequently, proliferation was quantified using a BrdU ELISA kit (NeoBioscience, Shenzhen, China). The optical density (OD) values were measured in an ELISA reader (Model 680, BIO-RAD, Hercules, California, USA) at 450 nm (reference wavelength: 690 nm).
Enzyme-Linked Immunosorbent Assay (ELISA)
Blood samples collected by cardiac puncture were left at room temperature for 2 h before centrifugation. Levels of IL-4, IL-10, IFN-γ, and TGF-β in the supernatants of MLR cultures and serum were detected using commercially available ELISA kits (NeoBioscience Technology Limited Company, Shenzhen, China). The analysis was performed in duplicate according to the manufacturer’s instruction.
Flow Cytometry
For testing the heterogenetic antibody isotype, serum was collected at different times (2 days and 7 days) in each group. C57/B6 splenic T cells (1 × 106 cells/100 μl) were incubated with 10 μl of recipient serum at 4 °C for 30 min. After washing twice in PBS, the cells were incubated with PE-anti-IgM, FITC-anti-IgG1 (R1-12D10, eBioscience, San Diego), or FITC-anti-IgG2a (R2a-21B2, eBioscience, San Diego) at 4 °C for 30 min.15 T lymphocytes from lymph nodes of recipient rats (1 × 106 cells/100 μl) were stained using fluorescently labeled antibodies in accordance with the manufacturer’s instructions. Antibodies used for flow cytometric analysis were anti-rat IFN-γ eFluor660 (DB-1, eBioscience, San Diego), anti-rat IL-4 eFluor660 (ox-81, eBioscience), anti-rat CD45R PE (HIS24, eBioscience, San Diego), anti-rat CD4 FITC (OX35, eBioscience), and anti-rat Foxp3 PE (FJK-16S, eBioscience, San Diego). The number of propagated cells used per tube ranged from ~15 000 to 20 000 of which 2000 cells were analyzed inside the lymphocyte gate. The stained cells were analyzed by FACS (Partec, Munster, Germany), and the data were analyzed using Flow Jo software (Tree Star, Ashland, OR).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from grafts of recipient mice using Trizol Reagent (Invitrogen, Carlsbad, CA) as instructed by the manufacturer. After removal of potentially contaminating DNA with DNase I, 1 μg of total RNA from each sample was reverse transcribed to the first strand cDNA with oligo (dT) 18 primer and M-MuLV reverse transcriptase (MBI Fermentas, ON, Canada). The primer sequences were as follows: IL-4 (forward: 5′-CACCTTGCTGTCACCCTG-3′, reverse: 5′-CGTAAGGACGTCTGGTAC-3′), IL-10 (forward: 5′-CTCAGCACTGCTATGTTGC-3′, reverse: 5′-CAGCAGTATGTTGTCCAGC-3′), IFN-γ (forward: 5′-GGATGCATTCATGAGCATCG-3′, reverse: 5′-CAGCACCGACTCCTTTTCC-3′), Foxp3 (forward: 5′-CCACACCTCCTCTTCTTCC-3′, reverse: 5′-GTGGTCTGTCCTGGAGAAG-3′). Each gene expression was normalized with GAPDH mRNA content. R-β-actin (forward: 5′-GGTCAGAAGGACTCCTACG-3′, reverse: 5′-CACACGCAGCTCATTGTAG-3′).
Statistical Analyses
Samples were assayed in duplicate or triplicate, and results are given as the mean±standard deviation from one representative experiment of at least three experiments. The mean survival time (MST) of each group was determined using the Kaplan–Meier method and the log-rank test. Data from the MLR, ELISA, and qRT-PCR were analyzed using the one-way analysis of variance. A P value<0.05 was considered to be a statistically significant; P<0.01 and P<0.001 indicated highly significant differences. All analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA).
RESULTS
Addition of IL-33 Further Prolongs Lef-Mediated Graft Survival
The median survival of grafts (time to rejection) in the untreated control rat group was 2.8±0.4 days. Lef-only treatment increased the median survival to 7.2±0.9 days, whereas IL-33 treatment alone did not affect the graft survival (2.5±0.5 days), compared to the control group. However, heart grafts in rats receiving IL-33 in combination with Lef survived for 17.3±2.3 days. The Kaplan–Meier plot (Figure 1) revealed that the graft loss in the Lef-only-treated rats mainly occurred between days 6 and 8, whereas all xenografts of the combination group were still beating during this time period. The survival of the combination group was prolonged by two times, compared with that of the Lef-only treatment group.
Pathological Examination of Cardiac Xenografts
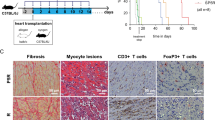
To determine the influence of IL-33 and Lef on cardiac xenograft survival, xenografts collected from Lewis rat recipients of the combination treatment exhibited markedly reduced inflammatory cell infiltration and greater tissue areas of normal myocardium on day 2 and day 7 (Figure 2a and b). On day 2 and day 7 post transplantation, we assessed the ISHLT scores of the hearts (Figure 2c). Grafts from the control group showed high levels of lymphocyte infiltration and severe tissue damage (scores ranged from 3 to 4), whereas those from the IL-33 alone treatment group had scores ranging from 1 to 3. Furthermore, grafts from the Lef and IL-33+Lef groups showed normal tissues (scores ranged from 0 to 2). Interestingly, on day 7 the extent of rejection/inflammation in the IL-33+Lef group was significantly lower than that of the Lef group (P<0.01). Staining of grafts in the IL-33+Lef group showed normal histological features, with scores between 1 and 2. All these findings revealed that treatement with IL-33 combined with Lef reduced the inflammatory cell infiltration, especially after day 7.
Pathological examination of cardiac xenografts and ISHLT scores. At day 2 post transplantation, heart grafts from the combination-treated group displayed reduced infiltration of mononuclear cells in the myocardial interstitium and preserved tissue architecture, compared to the untreated group. At day 7 post transplantation, xenografts from recipients treated with combination therapy had preserved myocardium with significantly reduced inflammatory infiltration. Results are representative of four animals in each group. (a, b: × 100, × 400) ISHLT scores and statistical analyses. Each dot represents the score from each section. (c) The horizontal line represents the mean for each group (n=6 in each group, **P<0.01, ***P<0.001).
IL-33 Treatment Affects Proportion and Function of T Cells in Recipient Spleens
On post-operative day 2 and day 7, splenic T cells were collected for the MLR assays. On day 2, the results showed that the proliferation of T lymphocytes from the IL-33+Lef group markedly decreased, compared with the control group (P<0.001) or Lef-only treatment group (P<0.001) (Figure 3a). Moreover, the OD value of the IL-33+Lef group was lower than that of the Lef-only treatment group after 7 days (P<0.05) (Figure 3b).
Proliferative responses of recipient splenic T cells to B6 donors in MLR assays. Each responder–stimulator combination was tested in three replicate wells, and each experiment was repeated three times (n=3, *P<0.05, **P<0.01, ***P<0.001; ^^^P<0.001, *means each group was compared to the control group; ^means each group was compared with each other in exclusion of the control group).
IL-33+Lef Treatment Decreases Th1 Cells and Cytokine Expression of Th1 T Cells in Xenograft Recipients
To evaluate the role of IL-33 in the expression of Th1 and Th2 Cells, T lymphocytes from lymph nodes of recipient rats were stained using fluorescently labeled antibodies and analyzed by flow cytometry. Consistent with the report by Yin et al,12 IL-33 administration to recipient rats decreased the incidence of CD4+IFN-γ+ (Th1) cells. On day 2, Th1 cells of the IL-33+Lef group decreased to 2.37%, compared with the control (6.47%) or Lef-only (5.50%) group (Figure 4a). Notably, on day 7 the proportion of CD4+IFN-γ+ (Th1) cells in recipients treated with IL-33+Lef (2.49%) was significantly lower when compared with those treated with only Lef (7.69%) (Figure 4a, lower). Previous work had implicated IL-4 as a strong inducer of Th2-type cytokines, and IFN-γ as a potent inducer of Th1-type immunity. Dujovny et al.16 also reported that IL-4 and IL-10 could decrease Th1 cytokine levels. To further support our contention that IL-33+Lef treatment suppressed the activation and function of Th1 cells, we investigated the cytokine expression of these lymphocytes isolated from spleens of recipient rats on post-operative day 2 and day 7. The proportion of CD4+ IFN-γ+ (Th1) cells in the IL-33+Lef group was decreased on day 2, compared to the control group and Lef group (Figure 4b, ***P<0.001, ^P<0.05). Meanwhile, no difference was observed between the IL-33 and IL-33+Lef group on day 2 (Figures 4b). However, the addition of IL-33 to Lef treatment resulted in a significantly smaller proportion of CD4+IFN-γ+ (Th1) cells, compared to Lef treatment only on day 7 (Figures 4b, **P<0.01). By contrast, the proportion of CD4+IL-4+ (Th2) cells had a marked increase in recipients treated with IL-33 when compared with the control, Lef or IL-33+Lef treatment group on day 2 (Figure 5a, first row, **P<0.01). No statistically significant difference was seen between the IL-33+Lef treatment and Lef treatment groups on day 7 (Figure 5b, first row).
IL-33+Lef treatment decreases Th1 cells and cytokine expression of Th1 T cells in xenograft recipients. IL-33-treated rats displayed decreased splenic CD4+IFN-γ+ (Th1) cells. However, IL-33 increased the proportion of CD4+Foxp3+ Tregs. CD4+IFN-γ+ (Th1) cells were analyzed by flow cytometry as a proportion of splenic lymphocytes. Results are means±standard deviation from one representative experiment of at least three experiments. On day 2 and day 7, IFN-γ levels in the supernatants of the MLR assay and serum were detected by using commercially available ELISA kits. Each reaction was carried out in triplicate. (*P<0.05, **P<0.01, ***P<0.001; ^P<0.05, *means each group was compared to the control group; ^means each group was compared with each other in exclusion of the control group). CD4+Foxp3+ Tregs were analyzed as a proportion of lymphocytes from the lymph nodes by flow cytometry.
IL-33+Lef treatment changes cytokine expression of xenograft recipients in splenocytes. On day 2 and day 7, levels of IL-4, IL-10, IFN-γ, and TGF-β in the supernatants from the MLR assay (a,b) and serum (c,d) were detected by using commercially available ELISA kits. Each reaction was carried out in triplicate. (*P<0.05, **P<0.01, ***P<0.001; ^P<0.05, ^^P<0.01, ^^^P<0.001, *means each group was compared to the control group; ^means each group was compared with each other in exclusion of the control group).
In the serum, we observed no statistically significant difference of IL-4 among the four groups on day 2 (Figure 5c, first row), but a tendency of decrease could be observed in the combination group. Interestingly, we found the same trend in spleens of recipient rats, as the addition of IL-33 to the Lef treatment resulted in a significantly smaller ratio of CD4+IFN-γ+ (Th1) cells, compared to those with Lef treatment only on day 7 (Figures 4b, P<0.01).
IL-33+Lef Treatment Changes Cytokine Expression of Xenograft Recipients in Splenocytes
We also examined levels of IFN-γ, IL-4, IL-10, and TGF-β in culture supernatants of splenocytes and serum of recipients. These cells were cultured and analyzed for cytokine production by ELISA. In both culture supernatants and serum, we found significantly decreased IFN-γ levels in the IL-33+Lef treatment group compared with Lef-only treatment group on day 7 (P<0.01) (Figure 4b). The IFN-γ levels of the IL-33+Lef treatment group was decreased, compared with the other three groups on day 2 in culture supernatants (control vs IL-33+Lef, P<0.001; Lef vs IL-33+Lef, P<0.05) (Figure 4b), but no differences on day 2 were found in the serum.
In addition, we tested levels of IL-4 to survey the function of IL-33 in promoting Th2-type responses. An obvious increase in IL-4 was observed in the IL-33-treated group, compared to the other three groups in the culture supernatant and serum on day 2 (P<0.01, IL-33 vs control, Lef or Lef+IL-33) (Figures 5a and 6c, first row), but no significant difference was seen on day 7 between the IL-33 and IL-33+Lef groups (Figures 5b and d, first row). To further verify the negative regulatory role of IL-33 in xenotransplantation, we tested the level of IL-10 and found that its production in culture supernatants and serum was strongly up-regulated in the IL-33+Lef treatment group, compared to the Lef treatment group on day 7 (P<0.01, P<0.05) (Figure 5b and d, second row), and the level of IL-10 from culture supernatants in the combination group was increased, compared to the other three groups on day 2 (P<0.05) (Figure 5a, second row). However, no differences in IL-10 levels were observed on day 2 in the serum (Figure 5d, second row). Interestingly, we found that the IL-33+Lef treatment group had up-regulated levels of TGF-β on day 2 in both the culture supernatants and serum (Figure 5a and c, third row), but the production of TGF-β was decreased on day 7 (Figure 5b and d, third row).
Flow cytometric analysis of CD4+Foxp3+ Tregs as a proportion of lymphocytes from lymph nodes. By qRT-PCR analysis of mRNA extracted from cardiac xenografts, relative expression levels of Foxp3+ were increased on day 2 in the combination group, compared with the control group (*P<0.05) and on day 7 in the combination group, compared with the Lef group (P<0.05).
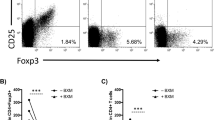
IL-33-Mediated Xenograft Survival is Associated with CD4+Foxp3+ Tregs
We suspected that Tregs may have an important role in prolonged xenograft survival after IL-33 therapy. Indeed, increased CD4+Foxp3+ Tregs in the IL-33-treated groups were detected (Figure 6). Higher percentages of CD4+Foxp3+ Tregs in IL-33-treated groups (8.40% in IL-33 treatment group, 9.31% in IL-33+Lef treatment group) were found, compared to the control and Lef treatment groups on day 2 (Figure 6a). Interestingly, transplants from IL-33+Lef-treated animals displayed a marked increase in CD4+Foxp3+ Tregs on day 7 (7.83 vs 12.3%) (Figure 6a). We next observed the expression of genes related to xenograft rejection by qRT-PCR analysis of mRNA extracted from cardiac xenografts. As shown in Figure 6b, relative expression levels of Foxp3+ cells in the combination group was increased on day 2, compared with the control group (P<0.05) and on day 7, compared with the Lef group (P<0.05).
IL-33 Affects Humoral Immunity in Transplant Recipients
To survey the effect of IL-33 treatment on B cells, which are also important in the setting of xenotransplantation rejection, we analyzed the animal lymph nodes by flow cytometry for changes in B-cell numbers. On day 2, only a slight decrease in the number of CD-45+B cells after IL-33 therapy was found (Figure 7a, first row). On day 7, a significant decrease was observed in the number of CD-45+B cells in the IL-33+Lef treatment group when compared to the Lef treatment group (from 22.0 to 12.1%) (Figure 7a, second row). To test if the reduction in B-cell numbers that was detected in four independent experiments led to decreased antibody-mediated rejection, serum samples of recipients were stained for IgG1, IgG2a, and IgM. As shown in Figure 7b–d, recipient rats treated with IL-33 had lower IgG2a, but no difference in IgG1 and IgM levels, when compared with the control or Lef-only treatment group on day 2.
DISCUSSION
Cytokines of the IL-1 family, such as IL-1 α/β and IL-18, have important roles in host defense, immune regulation, and inflammation.17 As a novel IL-1 family member, IL-33 has been shown to be functionally important in the induction and effects of type-2 immune responses.18 Th2-type cytokines have been reported to efficiently suppress cell-mediated immune responses.12 New studies indicate that IL-33 is a multifunctional protein that acts as transcriptional/signaling repressor, functions as an alarming alerting the immune system to necrosis, as well as serves as a cytokine that targets cells expressing ST2, the IL-33 receptor. Moreover, IL-33 is also regarded as a pleiotropic cytokine.5 Recent evidence in autoimmune disease and transplantation models associated tolerance induction with immune bias towards predominantly Th2 cell and cytokine function in the target organ, as well as inhibition of Th1 activity.19 Insight into biological functions of Th2 cytokines have led to novel therapeutic approaches to treat human inflammatory diseases. Many studies have demonstrated that promoting the Th2 profile could reduce the pathological progression of Th1-mediated colitis.20 Consistent with the results above, Turnquist et al4 reported that IL-33 monotherapy after a fully allogeneic mouse heart transplantation could significantly extend the graft survival that was associated with increased Th2-type responses and decreased systemic CD8+IFN-γ+ cells. Moreover, the IL-33 receptor ST2 is preferentially expressed on Treg cells, where it promotes Treg function and adaptation to the inflammatory environment,6 and IL-33 administration has been well-documented to cause an ST2-dependent expansion of suppressive CD4+ Foxp3+ Tregs.4 To our knowledge, the current study is the first to report the novel observation that during mouse-to-rat xenotransplantation, IL-33 could prolong xenograft survival through reducing the Th1-type cytokine IFN-γ, as well as expanding CD4+Foxp3+ Tregs.
Turnquist et al4 and Yin et al12 reported that IL-33 could prolong murine cardiac allograft survival. Consistent with those results, we found that the combination therapy of IL-33 and Lef following heart transplantation resulted in a significant prolonging of graft survival in concordant heart transplantation. In rats receiving the combination therapy, the median survival of heart grafts was twice as long as that of rats given Lef-only. Davidson et al21 reported that exogenous IL-13 alone could significantly improve allograft survival through repressing the proinflammatory cytokines IL-1 and TNF. Moreover, markedly reduced inflammatory cell infiltration and greater areas of normal myocardium were observed in the IL-33+ Lef treatment group, compared to other groups, especially on day 7.
Many studies have demonstrated that IL-33 amplifies Th2-type responses by up-regulating production of IL-4, IL-5, and IL-13.17, 22 Furthermore, substantial support exists for the hypothesis that a potent Th1 cytokine response is critical for the normal process of allograft rejection.23 Th1-type cytokines, such as IFN-γ, promote the generation of cellular immune responses and IgG2a antibodies, whereas Th2-type cytokines promote IgE and IgG1 production. However, IL-4 and IL-10 are known to decrease the levels of Th1 cytokines.16, 24 The absence of IFN-γ significantly reduced IgG response, specific suppression of IgG2a, IgG2b, IgG3 not IgG1.16 In addition, a deviation in the Th1/Th2 balance and high expression of CD4+Foxp3+ Tregs may lead to immune tolerance.25, 26 In this study we observed that IL-33 administration to recipient rats decreased the incidence of CD4+IFN-γ+ cells, and this effect was more apparent on the 7th day. To further support our contention that IL-33+Lef treatment suppressed the activation and function of Th1 cells, we investigated cytokine expression of lymphocytes and observed that IFN-γ levels were markedly decreased after IL-33+Lef therapy. At the same time, IL-33 administration promoted the proliferation of IL-10, especially on post-operative day 7, although its effect on IL-4 was not obvious at that time. These findings identified that IL-33-treated rats produced higher levels of inhibitory cytokines, including IL-10 and IL-4, and lowered the Th1 proinflammatory cytokine IFN-γ. The study also found that IL-33 treatment affected the proportion and function of T cells in recipient spleens.
The development of tolerance to allografts is associated with levels of CD4+Foxp3+ Tregs that can inhibit the ability of normal alloreactive CD4+ and CD8+T cells to mediate rejection.27 In recent studies, flow cytometric analysis showed a direct induction of CD4+Foxp3+ Tregs lymph nodes after IL-33 therapy, which prolonged the survival of allografts.25 In this context, we observed that transplants from IL-33+Lef-treated animals displayed a marked increase in CD4+Foxp3+ Tregs on day 7 (7.83 vs 12.3%) (Figure 5a). Moreover, we observed a decrease in the number of CD-45+ B cells in the IL-33+Lef treatment group when compared to that of the Lef treatment group (Figure 7a, second row). Consistent with our observations, Brunner et al25 also reported that IL-33 therapy reduced the number of CD19+ B cells and alloantibodies in the circulation as well as on the allograft.
Dujovny et al16 reported that the absence of IFN-γ significantly reduced IgG responses, specifically suppressing IgG2a. The absence of IL-4 did not result in the total reduction of IgG titers, but suppressed IgG1. Consistent with that study, we also found that rats administered IL-33+Lef had a significant decrease in the level of IFN-γ, compared to Lef-only-treated rats. The level of IgG2a was also decreased in rats given IL-33 alone. Although IL-33-treated mice have been shown previously to produce more IgG1 and IgM isotype alloantibodies,12 no statistically differences in IgG1 and IgM levels were seen in our study, which may be related to the function of Lef.9, 28, 29
In summary, we have provided in this study the first evidence that IL-33 and Lef combination therapy following mouse-to-rat heart transplantation resulted in significant prolonging of graft survival, which was associated with markedly decreased Th1 cells and IFN-γ, and with increased IL-10 and CD4+Foxp3+ Tregs. These findings reveal previously undefined functions of IL-33 in xenotransplantation. Although the mechanistic basis of action of IL-33 in concordant heart transplantation requires further definition, these findings may facilitate development of a new strategy for organ transplantation, especially xenotransplantation.
References
Yan Y, Verbeken E, Yu L, et al. Effects of a short course of leflunomide on T-independent B-lymphocyte xenoreactivity and on susceptibility of xenografts to acute or chronic rejection. Transplantation 2005;79:135–141.
Cheng PP, Liu XC, Ma PF, et al. iPSC-MSCs combined with low-dose rapamycin induced islet allograft tolerance through suppressing Th1 and enhancing regulatory T-cell differentiation. Stem Cells Dev 2015;24:1793–1804.
Biglarnia AR, Emanuelsson C, Quach M, et al. The free radical scavenger S-PBN significantly prolongs DSG-mediated graft survival in experimental xenotransplantation. Xenotransplantation 2012;19:166–176.
Turnquist HR, Zhao Z, Rosborough BR, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol 2011;187:4598–4610.
Liu Q, Turnquist HR. Implications for Interleukin-33 in solid organ transplantation. Cytokine 2013;62:183–194.
Schiering C, Krausgruber T, Chomka A et al, The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014;513:564–568.
Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide suppresses TNF-induced cellular responses: effects on NF- B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol 2000;165:5962–5969.
Hassikou H, El Haouri M, Tabache F, et al. Leflunomide-induced toxic epidermal necrolysis in a patient with rheumatoid arthritis. Joint Bone Spine 2008;75:597–599.
Sobhani K, Garrett DA, Liu DP et al, A rapid and simple high-performance liquid chromatography assay for the leflunomide metabolite, teriflunomide (A77 1726), in renal transplant recipients. Am J Clin Pathol 2010;133:454–457.
Chackerian AA, Oldham ER, Murphy EE, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol 2007;179:2551–2555.
Eyre DW, Babakhani F, Griffiths D, et al. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for preventing reinfection and relapse of infection with Clostridium difficile. J Infect Dis 2014;209:1446–1451.
Yin H, Li XY, Jin XB et al, IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation 2010;89:1189–1197.
Laumonier T, Mohacsi PJ, Matozan KM et al, Endothelial cell protection by dextran sulfate: a novel strategy to prevent acute vascular rejection in xenotransplantation. Am J Transplant 2004;4:181–187.
Wang Q, Liu Y, Li XK. Simplified technique for heterotopic vascularized cervical heart transplantation in mice. Microsurgery 2005;25:76–79.
Lin Y, Dai H, Su J, et al. Arsenic trioxide is a novel agent for combination therapy to prolong heart allograft survival in allo-primed T cells transferred mice. Transpl Immunol 2011;25:194–201.
Dujovny N, Varghese A, Shen J et al, Acute xenograft rejection mediated by antibodies produced independently of TH1/TH2 cytokine profiles. Am J Transplant 2002; 526–534.
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–490.
Mirchandani AS, Salmond RJ, Liew FY. Interleukin-33 and the function of innate lymphoid cells. Trends Immunol 2012;33:389–396.
Sayegh MH1 AE, Hancock WW, Russell ME et al, CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med 1995; 1869–1874.
Daniel C, Sartory NA, Zahn N, et al. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 2008;324:23–33.
Davidson C, Verma ND, Robinson CM, et al. IL-13 prolongs allograft survival: association with inhibition of macrophage cytokine activation. Transpl Immunol 2007;17:178–186.
Farahani R, Sherkat R, Hakemi MG, et al. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res 2014;3:127.
Kupiec-Weglinski JW1 WB, Papp I, Schmidbauer G et al, CD4 mAb therapy modulates alloantibody production and intracardiac graft deposition in association with selective inhibition of Th1 lymphokine. J Immunol 1993;151:5053–5061.
He X, Wu W, Lu Y et al, Effect of interleukin-33 on Th1/Th2 cytokine ratio in peripheral lymphocytes in asthmatic mice. Chin Med J (Engl) 2014;127:1517–1522.
Brunner SM, Schiechl G, Falk W et al, Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int 2011;24:1027–1039.
Li B, Tian L, Diao Y, et al. Exogenous IL-10 induces corneal transplantation immune tolerance by a mechanism associated with the altered Th1/Th2 cytokine ratio and the increased expression of TGF-β. Mol Med Rep 2014; 2245–2250.
Hall BM1 PN, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med 1990;171:141–157.
Chong AS1 ML, Yin D, Blinder L, et al. Tolerance of T-independent xeno-antibody responses in the hamster-to-rat xenotransplantation model is species-restricted but not tissue-specific. Xenotransplantation 2000;7:1.
Bersztel A, Lorant T, Bjorkland A et al, Antibody responses to xenogenic antigens—a study in the mouse-to-rat system. Tissue Antigens 2006;68:483–488.
Acknowledgements
This work was supported by grants from the Major State Scientific Research Program of China (No. 2012CBA01303) and the National Natural Science Foundation of China (81301786).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dai, C., Lu, FN., Jin, N. et al. Recombinant IL-33 prolongs leflunomide-mediated graft survival by reducing IFN-γ and expanding CD4+Foxp3+ T cells in concordant heart transplantation. Lab Invest 96, 820–829 (2016). https://doi.org/10.1038/labinvest.2016.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.54
This article is cited by
-
IL-33/ST2 signaling in liver transplantation
Cellular & Molecular Immunology (2021)
-
Arsenic Trioxide Combining Leflunomide Activates Nrf2-ARE-HO-1 Signaling Pathway and Protects Heart Xenografts
Chinese Journal of Integrative Medicine (2021)
-
Regulatory T cell frequency, but not plasma IL-33 levels, represents potential immunological biomarker to predict clinical response to intravenous immunoglobulin therapy
Journal of Neuroinflammation (2017)