Abstract
Cancer cells with tumorigenic potential are limited to a small subpopulation known as cancer-initiating cells (CICs). Recently we investigated a candidate of CICs of lymphoplasmacytic lymphoma (LPL), which is positive for both B-cell marker CD20 and plasma-cell marker CD138. We reported that the subpopulation of CD20− CD138− phenotype, in which both markers were negative was a candidate of CICs in LPL using LPL cell line, MWCL-1. CICs are known to be plastic under stressed condition, in which non-CICs are changed to CICs. In the present study, we investigated the plasticity of CICs of LPL, and found that hypoxia induced the conversion of CD20+ CD138− to CD20− CD138− phenotype. We then searched for markers preferentially expressed in CD20− CD138− subpopulation, and the chemokine receptor CXCR7 was isolated. When cultured with CXCL12, a ligand of CXCR7, the number of CD20− CD138− cells increased in a time- and dose-dependent manner. In addition, hypoxia enhanced the expression level of CXCL12 in MWCL-1. In clinical samples of LPL, a few tumor cells expressed CXCR7, in which CD20 expression was not detected. These results indicated that hypoxia and CXCL12-CXCR7 axis appeared to be advantageous microenvironments to CD20− CD138− cells.
Similar content being viewed by others
Main
Tumors derive from a single clone, but consist of heterogenous cell subpopulations whose features and functions are diverse. It has been demonstrated that tumorigenic potential is limited to a small subpopulation known as cancer-initiating cells (CICs).1, 2, 3, 4, 5, 6, 7, 8, 9, 10 CICs are one of the causes of tumor recurrence, because CICs efficiently escape apoptosis by effluxing anti-tumor drugs and degrading reactive oxygen species (ROS) that are related to radiation-induced apoptosis.9, 10, 11 CICs could be effective targets for therapeutic intervention. On one hand, CICs yield both CICs and non-CICs; on the other hand, non-CICs rarely yield CICs.1, 2, 9, 10 However, the plastic dedifferentiation of non-CICs to CICs has been recently noticed.12, 13 The rate of spontaneous conversion of non-CICs to CICs might be very low and the kinetics of spontaneous dedifferentiation might be slow, but the plasticity of non-CICs occurs more prevalently under stressed conditions.12 For example, culturing cancer cells under hypoxic condition increases the expression of stemness genes and the percentage of phenotypic CICs.14, 15, 16
Waldenström macroglobulinemia (WM) is defined as lymphoplasmacytic lymphoma (LPL) with bone marrow involvement and an immunoglobulin M (IgM) monoclonal gammopathy.17, 18 LPL is an indolent non-Hodgkin’s lymphoma and consists of tumor cells with relatively diverse surface markers. LPL shows a spectrum of small B lymphocytes, plasma cells and lymphoplasmacytoid cells.18, 19 In addition to the expression of soluble IgM and the pan B-cell antigens such as CD20, LPL cells coexpress the markers of mature plasma cells such as CD138.18, 20, 21 Recently, LPL cell line, called MWCL-1, has been established. MWCL-1 has a rigorous clonal relationship with the primary LPL, which retains similar immunophenotypic and biological properties of the initial tumor clone.22 Because of the heterogenous phenotypes of lymphoma cells and the establishment of cell line, LPL is suitable for the study of CICs in malignant lymphomas. Using MWCL-1 cell line, we found that the CD20− CD138− double-negative subpopulation lacking B lymphocyte and plasma-cell markers was a candidate of CICs of LPL, because CD20− CD138− double-negative subpopulation was resistant to apoptosis, showed high ROS-expelling and in vitro colony formation activities, and yielded all subpopulations (CD20+ single-positive, CD20+ CD138+ double-positive, and CD20− CD138− double-negative).23
In the present study, using MWCL-1 cell line and LPL clinical samples, we found that C-X-C chemokine receptor (CXCR) 7 (also known as GPCR RDC1) was highly expressed in CD20− CD138− subpopulation of MWCL-1, and that hypoxia and CXCR7-mediated signal were closely related to increase of CD20− CD138− subpopulation.
MATERIALS AND METHODS
Cell Line and Sorting
WM cell line, MWCL-1 was provided from Mayo Foundation for Medical Education and Research. MWCL-1 cells were cultured in IMDM+GlutaMAX-I (Gibco by Life Technologies, Carlsbad, USA) supplemented with 10% fetal bovine serum (Central America Origin, Biosera, Kansas City, USA). Cells were stained with CD20-Allophycocyanin (APC) (clone 2H7, BD Biosciences, San Jose, USA) and CD138-Phycoerythrin (PE) (clone MI15, BD Biosciences) antibodies, and CD20− CD138−, CD20+ CD138−, CD20+ CD138+ subpopulations were sorted with FACS Aria II (BD Biosciences). We reacted cells in the presence of human FcR-blocking reagent (Miltenyi Biotec, Auburn, USA). The dot blot pattern obtained with FcR blocking was comparable to that without blocking.23 To remove dead cells, propidium iodide (PI) staining and gating of forward-scatter-area (FSC-A) versus side-scatter-area (SSC-A) were performed. The proportion of dead cells removed by PI staining and gating of FSC-A versus SSC-A was almost same as that removed only by gating of FSC-A versus SSC-A.23 Then, in the following experiments, FcR blocking and PI staining were omitted. As negative controls, cells were left unstained.
Culture in Hypoxic versus Normoxic Conditions
Unsorted or sorted CD20+ CD138− cells were cultured in hypoxic (O2: 1%) versus normoxic (O2: 20%) conditions, and their immunophenotypes of CD20 and CD138 were examined with FACS Aria II or FACS Canto II (BD Biosciences). As negative controls, cells were left unstained. Data were analyzed by Cell Quest software (BD Biosciences).
Microarray Analysis
Microarray analysis was performed as described previously.24 Briefly, the microarray platforms used in this study were Whole Human Genome Microarray 4x44K version 2 (G4845A) (Agilent Technologies, Santa Clara, CA). In total, 200 ng of total RNA derived from 1.4 × 106 sorted CD20− CD138− or CD20+ CD138+ cells were extracted using a miRNeasy kit (Qiagen, Venlo, The Netherlands) and then reverse transcribed and labeled using a low input Quick Amp labeling kit (Agilent) following the manufacturer's instructions. Raw data were imported into GeneSpring GX 11.0 (Agilent) for database management and quality control. The fold change values were calculated as the ratio of the normalized value between CD20− CD138− and CD20+ CD138+ cells. The details of the microarray data have been deposited in the Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo) database (accession number GSE73756).
Immunoblotting
Cells were lysed using the buffer containing 10 mM Hepes, 10 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.1% Nonidet P-40 and 10% protease inhibitor cocktail (Sigma-Aldrich, St Louis, USA). Electrophoresis was performed with 10% sodium dodecyl sulfate–polyacrylamide gels (ATTO, Tokyo, Japan) and proteins were transferred to polyvinylidene fluoride membranes (Merck Millipore, Billerica, USA). CXCR7 antibody (ab137485, Abcam, Tokyo, Japan, dilution at 1:1000), CXCR4 (also known as CD184) antibody (ab2074, Abcam, Tokyo, Japan, dilution at 1:1000) and α-actin antibody (Sigma-Aldrich, dilution at 1:1000) were used as the primary antibodies. HRP-conjugated anti-rabbit IgG (H+L chain) (MBL, Nagoya, Japan, dilution at 1:2000) was used as the secondary antibody. Data were analyzed by Image Lab software (Bio-Rad, Hercules, USA).
Culture after Addition of CXCL12
Cells were cultured after addition of CXCL12 (the ligand of CXCR7, also known as stromal cell-derived factor-1α) (Sigma-Aldrich, concentration at 100 or 300 ng/ml) under normoxic or hypoxic conditions, and their immunophenotypes of CD20 and CD138 were examined with FACS Aria II or FACS Canto II (BD Biosciences). As negative controls, cells were left unstained. Data were analyzed by Cell Quest software (BD Biosciences).
Assay for Intracellular CXCL12 Level
Multicolor staining for the cell surface antigens, CD20-APC and CD138-PE, and the intracellular chemokine, CXCL12-Carboxyfluorescein (clone 79018, R&D Systems, Minneapolis, USA) was performed with BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) as manufacturer’s instructions. We examined intracellular CXCL12 levels of CD20− CD138−, CD20+ CD138− and CD20+ CD138+ subpopulations in MWCL-1 with FACS Aria II or FACS Canto II (BD Biosciences). As negative controls, cells were stained with matched isotype control, mouse IgG1 isotype control-Fluorescein (clone 11711, R&D Systems) antibody.
Immunohistochemistry
CXCR7 expression was immunohistochemically examined in clinical cases of LPL diagnosed in Osaka University Hospital from 2002 to 2013. The clinicopathological features were shown in Table 1. Histological specimens were fixed in 10% formalin and routinely processed for paraffin-embedding. Paraffin-embedded samples were stored in a dark room at room temperature. Sections were cut into 4 μm thickness. After antigen retrieval with Pascal pressurized heating chamber (DAKO), samples were incubated with CD20, CD79a, CD3, CD138 (DAKO, mouse monoclonal antibodies diluted at 1:400, 1:100, 1:50, and 1:50, respectively) and CXCR7 (ab137485, Abcam, rabbit polyclonal antibody diluted at 1:150) antibodies. Then, samples were treated with ChemMate EnVision kit (DAKO). DAB (DAKO) was used as a chromogen. Double staining of CD20 and CXCR7 was also performed using Alexa Fluor 568 goat anti-mouse IgG (H+L) (Invitrogen, Carlsbad, USA, dilution at 1:1000) and Alexa Fluor 488 goat anti-rabbit IgG (H+L) (Invitrogen, Carlsbad, USA, dilution at 1:2000). CD20 was stained with red color and CXCR7 with green color. The study was approved by the institutional review board for clinical research at Osaka University Hospital (no. 12461).
Statistical Analysis
Statistical analyses were performed using t-tests. The values are shown as the mean±s.e.m. of three experiments. The P-values <0.05 were considered to be statistically significant.
Results
Effect of Hypoxic Condition on CD20− CD138− Subpopulation
The rate of spontaneous conversion of non-CICs to CICs is very low, but its conversion has been reported to occur more prevalently under stressed conditions.12 To examine the conversion to CD20− CD138− subpopulation under stressed condition, unsorted MWCL-1 cells were cultured in hypoxia (O2: 1%). The proportion of CD20− CD138− cells gradually increased as compared with that cultured in normoxic condition (Figure 1). Then, the conversion of sorted CD20+ CD138− cells to CD20− CD138− phenotype under hypoxic condition was examined. Sorted CD20+ CD138− cells yielded CD20− CD138− cells in hypoxic but not in normoxic condition (Figure 2).
Immunophenotypes of unsorted MWCL-1 cultured in hypoxic (O2: 1%) condition. Expression of CD20 and CD138 was examined. The proportion of the number of CD20− CD138− cells gradually increased with the lapse of hypoxic time as compared with that cultured in normoxic (O2: 20%) condition. The proportion values of numbers of CD20− CD138− cells were shown.
High CXCR7 Expression in CD20− CD138− Subpopulation
Because no useful positive markers expressed in CD20− CD138− cells have been identified, we conducted DNA microarray-based comparative analyses between sorted CD20− CD138− and CD20+ CD138+ cells. In the analysis, 653 out of 34 127 probes showed greater than two-fold changes in expression (top 25 genes were listed in Table 2). From the result that the number of CD20− CD138− cells increased under hypoxia, we searched for molecules whose expression is enhanced in hypoxic condition. Among the top 25 molecules, only CXCR7 expression level is enhanced under hypoxic condition in many reports (http://www.hypoxiadb.com/). CXCL12-CXCR4/CXCR7 signaling is known to be important in hematopoietic stem cell maintenance in bone marrow,25 and we further examined CXCR7 in CD20− CD138− cells. To confirm the high expression of CXCR7 in CD20− CD138− subpopulation, we carried out immunoblotting for CXCR7 with sorted CD20− CD138−, CD20+ CD138− and CD20+ CD138+ cells. CXCR7 protein expression level was the highest in CD20− CD138− subpopulation (Figure 3). CXCL12 is the ligand of CXCR7, binds to CXCR4 as well as CXCR7, and both receptors are closely related according to reported modes of CXCL12-CXCR4/CXCR7 signaling.26 Then, the expression level of CXCR4 was also examined in sorted CD20− CD138−, CD20+ CD138− and CD20+ CD138+ cells. In contrast to CXCR7, the expression level of CXCR4 was low and comparable among examined subpopulations (Figure 3).
Immunoblotting for CXCR7. (a) Chemiluminescent blot bands for CXCR7 and CXCR4 were detected with sorted CD20− CD138−, CD20+ CD138− and CD20+ CD138+ cells. Actin was detected by re-blotting with the same sample set. (b) Bar graphs showing the relative intensity of proteins analyzed by Image Lab software. CXCR7 protein expression of CD20− CD138− subpopulation was the highest.
Effect of CXCL12 on CD20− CD138− Subpopulation and the Intracellular CXCL12 Amount under Hypoxia
To examine whether the stimulation of CXCR7 exerted some influence, CXCL12 was added and the change of proportion of CD20− CD138− subpopulation was observed. When cultured with CXCL12, the proportion of CD20− CD138− cells gradually and dose-dependently increased (Figures 4a and b). Next, CXCL12 was added under hypoxic condition. Under hypoxia, the proportion of CD20− CD138− cells increased significantly even in the absence of CXCL12, and no further increase was detected when CXCL12 was added (Figures 5a and b). Then, we supposed that the level of endogenous CXCL12 increased under hypoxia, which enhanced the proportion of CD20− CD138− cells. To confirm this, under hypoxic condition, intracellular CXCL12 levels were examined in CD20− CD138−, CD20+ CD138−, and CD20+ CD138+ subpopulations. Hypoxia induced the production of intracellular CXCL12 in all subpopulations (Figure 6). Especially CD20+ CD138+ subpopulation showed a marked expression of CXCL12 at 5 and 24 h after the initiation of hypoxic culture (Figure 6).
Immunophenotype of MWCL-1 in the presence of CXCL12 (100 or 300 ng/ml) under normoxia (O2: 20%). (a) Immunophenotypes of MWCL1 were examined. The representative proportion values of numbers of CD20− CD138− cells were shown. (b) Bar graphs showing the proportion of number of CD20− CD138− cells. The values are the average±s.e.m. based on the results of three analyses. When cultured with CXCL12, the proportion of number of CD20− CD138− cells time- and dose-dependently increased (*P<0.05, **P<0.01).
Immunophenotype of MWCL-1 in the presence of CXCL12 (300 ng/ml) under hypoxia (O2: 1%). (a) MWCL-1 cells were cultured under hypoxia for 24 hours with or without CXCL12, and their immunophenotypes were examined. The representative proportion values of numbers of CD20− CD138− cells were shown. (b) Bar graphs showing the proportion of number of CD20− CD138− cells. The values are the average±s.e.m. based on the results of three analyses (**P<0.01).
Intracellular CXCL12 levels examined in CD20− CD138−, CD20+ CD138−, and CD20+ CD138+ subpopulations under hypoxic (O2: 1%) condition. The gray area corresponds to negative controls; cells were stained with matched isotype control. Hypoxia induced the production of intracellular CXCL12 in all subpopulations.
CXCR7 Expression in Clinical Samples of LPL
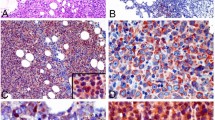
Finally, CXCR7 expression was examined in clinical samples of LPL. Immunohistochemically CXCR7 was expressed in a few tumor cells in seven out of eight clinical samples of LPL (Figure 7a and Table 1). Double staining of CD20 and CXCR7 revealed CXCR7 expressing cells did not express CD20 (Figures 7b and d). Unlike T-cell lymphoma and Hodgkin’s lymphoma, LPL is composed of monomorphous tumor cells, most of which are CD20+. Since CXCR7+ cells were surrounded by a lot of CD20+ lymphoma cells, we considered that CXCR7+ cells were a member of lymphoma cells.
Immunohistochemical staining of CXCR7 and CD20 in clinical samples of LPL. Immunohistochemically CXCR7 was expressed in a few tumor cells in clinical samples of LPL (a, × 100). In double staining, CXCR7-positive cells were shown in green (b, × 600), and CD20-positive cells in red (c, × 600). Merged image was also shown (d, × 600). CXCR7-positive cells were CD20-negative. Representative case was shown.
Discussion
Few studies have been done on CICs of malignant lymphomas. We investigated a candidate of CICs of LPL using MWCL-1. In addition to the expression of the pan B-cell antigen such as CD20, LPL cells coexpress marker of mature plasma cells such as CD138. When stained with anti-CD20 and anti-CD138 antibodies, MWCL-1 cells were classified into three subpopulations; CD20− CD138−, CD20+ CD138−, and CD20+ CD138+. Among these three subpopuations, CD20− CD138− was a candidate of CICs of LPL, because CD20− CD138− yielded all three subpopulations and showed high in vitro colony formation, anti-apoptotic, and ROS-expelling activities.23 It is known that the plasticity of CICs is induced under stressed condition, in which non-CICs yield CICs.12 Here, we demonstrated that CD20− CD138− subpopulation was yielded from CD20+ CD138− subpopulation in hypoxia. This result suggested that the plasticity of LPL CICs was induced by hypoxia.
In our previous immunohistochemical study using clinical samples of LPL, most tumor cells were CD20 and/or CD138 positive.23 We supposed that it is difficult to figure out CD20− CD138− cells because of a lack of positive markers. Then, we tried to identify markers specifically expressed in CD20− CD138− cells by DNA microarray. Among genes highly expressed in CD20− CD138− cells, chemokine receptor CXCR7 was a unique gene whose expression level was enhanced under hypoxia in many reports. Indeed, the expression level of CXCR7 was higher in CD20− CD138− cells than other two subpopulations, CD20+ CD138− and CD20+ CD138+ cells. The addition of CXCL12, a ligand for CXCR7, increased the amount of CD20− CD138− cells. Hypoxia increased the expression level of CXCL12 in all subpopulations. These results indicated that the axis of CXCL12-CXCR7 was important for maintenance of CD20− CD138− cells. CXCL12 induced by hypoxia may increase the subpopulation of CD20− CD138−. Immunohistochemical analysis in clinical samples revealed that a small number of cells expressed CXCR7, in which CD20 expression was not detected. CXCR7 was supposed to be one of the useful positive markers expressing in a candidate of CICs of LPL.
The feature of LPL is that most cases involve bone marrow.18 CXCL12 is known to be essential for maintenance of hematopoietic stem cells, in which CXCL12-CXCR4 axis is important.25 According to the recent report by Roccaro et al,27 28% of LPL cases possessed C1013G mutation in CXCR4 gene, driving tumor progression and drug resistance. In our study on MWCL-1, the expression level of CXCR4 was low and comparable among CD20− CD138−, CD20+ CD138−, and CD20+ CD138+ cells. This was consistent with the recent report that the expression of CXCR4 was hardly detectable in MWCL-1.28 Recent report demonstrated that the signal from CXCR4 is common to that of CXCR7.26 Since not all LPL cases possessed driver mutation at CXCR4,27 several cases of LPL might utilize signals from CXCR7.
Seven out of eight clinical samples possessed CXCR7+ LPL cells, but one case did not. The case without CXCR7+ LPL cells showed aggressive behavior: extramedullary invasion and poor prognosis. Roccaro et al27 reported the aggressiveness of cases with C1013G driver mutation in CXCR4 gene. The case without CXCR7+ LPL cells might have such mutation. However, three cases showing extramedullary invasion contained CXCR7+ LPL cells in our study. Further investigation with more cases will be needed.
CXCR7 expression of normal peripheral blood cells was controversial. On one hand, it was reported that transformed cells but not normal peripheral blood leukocytes frequently express CXCR7.29, 30 On the other hand, it was reported that CXCR7 was expressed by monocytes, granulocytes, and platelets among peripheral blood cells.31 Although the discrepancy might be because of the different techniques employed to prepare cell samples, which could alter or destroy epitope recognition, the absence of CXCR7 on normal peripheral blood leukocytes was demonstrated with the most sensitive detection methods available.30 Most normal leukocytes expressed CXCR4.30 Therefore, CXCR7 might be better targets for therapeutic intervention with a few side effects than CXCR4 in some LPL cases.
Accession codes
References
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–737.
Reya T, Morrison SJ, Clarke MF et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111.
Al-Hajj M, Wicha MS, Benito-Hernandez A et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983–3988.
Lessard J, Sauvageau G . Bmi-1 determined the proliferative capacity of normal and leukaemic stem cells. Nature 2003;423:255–260.
Kondo T, Setoguchi T, Taga T . Persistence of a small subppulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA 2004;101:781–786.
Singh SK, Hawkins C, Clarke ID et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401.
O’Brien CA, Pollett A, Gallinger S et al. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature 2007;445:106–110.
Ricci-Vitiani L, Lombardi DG, Pilozzi E et al. Identification and expansion of human colon-cancer initiating cells. Nature 2007;445:111–115.
Ikeda J, Mamat S, Tian T et al. Tumorigenic potential of mononucleated small cells of Hodgkin lymphoma cell lines. Am J Pathol 2010;177:3081–3088.
Ikeda J, Mamat S, Tian T et al. Reactive oxygen species and aldehyde dehydrogenase activity in Hodgkin lymphoma cells. Lab Invest 2012;92:606–614.
Diehn M, Cho RW, Lobo NA et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458:780–783.
Tang DG . Understanding cancer stem cell heterogeneity and plasticity. Cell Res 2012;22:457–472.
Meacham CE, Morrison SJ . Tumour heterogeneity and cancer cell plasticity. Nature 2013;501:328–337.
Das B, Tsuchida R, Malkin D et al. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008;26:1818–1830.
Koh MY, Lemos R Jr, Liu X et al. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res 2011;71:4015–4027.
Mathieu J, Zhang Z, Zhou W et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res 2011;71:4640–4652.
Owen RG, Treon SP, Al-Katib A et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s macroglobulinemia. Semin Oncol 2003;30:110–115.
Swerdlow SH, Berger F, Pileri SA et al. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn. IARC Press: Lyon, 2008, pp 194–195.
Barakat FH, Medeiros J, Wei EX et al. Residual monotypic plasma cells in patients with Waldenstrom macroglobulinemia after therapy. Hematopathology 2011;135:365–373.
Konoplev S, Medeiros J, Bueso-Ramos CE et al. Immunophenotypic profile of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Am J Clin Pathol 2005;124:414–420.
Morice WG, Chen D, Kurtin PJ et al. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenstrom’s macroglobulinemia. Mod Pathol 2009;22:807–816.
Hodge LS, Novak AJ, Grote DM et al. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL-1. Blood 2011;117:e190–e197.
Wada N, Zhan M, Hori Y et al. Characterization of subpopulation lacking both B-cell and plasma cell markers in Waldenstrom macroglobulinemia cell line. Lab Invest 2014;94:79–88.
Funato Y, Terabayashi T, Sakamoto R et al. Nucleoredoxin sustains Wnt/β-catenin signaling by retaining a pool of inactive dishevelled protein. Curr Biol 2010;20:1945–1952.
Broxmeyer HE . Chemokines in hematopoiesis. Curr Opin Hematol 2008;15:49–58.
Puchert M, Engele J . The peculiarities of the SDF-1/CXCL12 system: in some cells, CXCR4 and CXCR7 sing solos, in others, they sing duets. Cell Tissue Res 2014;355:239–253.
Roccaro AM, Sacco A, Jimenez C et al. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood 2014;123:4120–4131.
Paulus A, Chitta KS, Wallace PK et al. Immunophenotyping of Waldenströms macroglobulinemia cell lines reveals distinct patterns of surface antigen expression: potential biological and therapeutic implications. PLoS One 2015;10:e0122338.
Mantovani A, Savino B, Locati M et al. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev 2010;21:27–39.
Berahovich RD, Zabel BA, Penfold ME et al. CXCR7 protein is not expressed on human or mouse leukocytes. J Immunol 2010;185:5130–5139.
Sánchez-Martín L, Sánchez-Mateos P, Cabañas C . CXCR7 impact on CXCL12 biology and disease. Trends Mol Med 2013;19:12–22.
Acknowledgements
We thank Professor Ansell SM, Mayo Foundation for Medical Education and Research, for providing MWCL-1, and Mr. Kohara M, Ms. Sawamura T, Nihei M, Maeno E, and Tsuruta Y for technical assistance and support. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (#T264604700, #T15K083630, #T254604350, and #T15K084230).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study describes the search for cancer-initiating cells in lymphoplasmacytic lymphoma. The authors discovered a potential candidate cell type, a hypoxia-induced subpopulation of cells that are CD20- CD138-. and overexpress the C-X-C chemokine receptor 7. Such cells may be targets for therapeutic intervention.
Rights and permissions
About this article
Cite this article
Wada, N., Ikeda, Ji., Nojima, S. et al. Requirement of CXCL12-CXCR7 signaling for CD20− CD138− double-negative population in lymphoplasmacytic lymphoma. Lab Invest 96, 517–525 (2016). https://doi.org/10.1038/labinvest.2016.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.28