Abstract
KRASG12D can cause lung cancer rapidly, but is not sufficient to induce thyroid cancer. It is not clear whether long-term serum thyroid stimulating hormone (TSH) stimulation can promote KRASG12D-mediated thyroid follicular cell transformation. In the present study, we investigated the effect of long-term TSH stimulation in KRASG12D knock-in mice and the role of Sprouty1 (SPRY1) in KRASG12D-mediated signaling. We used TPO-KRASG12D mice for thyroid-specific expression of KRASG12D under the endogenous KRAS promoter. Twenty TPO-KRASG12D mice were given anti-thyroid drug propylthiouracil (PTU, 0.1% w/v) in drinking water to induce serum TSH and 20 mice were without PTU treatment. Equal number of wild-type littermates (TPO-KRASWT) was given the same treatment. The expression of SPRY1, a negative regulator of receptor tyrosine kinase (RTK) signaling, was analyzed in both KRASG12D-and BRAFV600E-induced thyroid cancers. Without PTU treatment, only mild thyroid enlargement and hyperplasia were observed in TPO-KRASG12D mice. With PTU treatment, significant thyroid enlargement and hyperplasia occurred in both TPO-KRASG12D and TPO-KRASWT littermates. Thyroids from TPO-KRASG12D mice were six times larger than TPO-KRASWT littermates. Distinct thyroid histology was found between TPO-KRASG12D and TPO-KRASWT mice: thyroid from TPO-KRASG12D mice showed hyperplasia with well-maintained follicular architecture whereas in TPO-KRASWT mice this structure was replaced by papillary hyperplasia. Among 10 TPO-KRASG12D mice monitored for 14 months, two developed follicular thyroid cancer (FTC), one with pulmonary metastasis. Differential SPRY1 expression was demonstrated: increased in FTC and reduced in papillary thyroid cancer (PTC). The increased SPRY1 expression in FTC promoted TSH-RAS signaling through PI3K/AKT pathway whereas downregulation of SPRY1 by BRAFV600E in PTC resulted in both MAPK and PI3K/AKT activation. We conclude that chronic TSH stimulation can enhance KRASG12D-mediated oncogenesis, leading to FTC. SPRY1 may function as a molecular switch to control MAPK signaling and its downregulation by BRAFV600E favors PTC development.
Similar content being viewed by others
Main
Thyroid cancer is the most common type of endocrine malignancies and its incidence is rising rapidly in recently years, especially among women.1 Histologically, it can be classified into papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC). PTC is the most common type of differentiated thyroid carcinomas, accounting for more than 80% of thyroid cancer, and FTC accounts for 15%.2 The BRAFV600E is the most common genetic alterations in PTC with overall rate of 44%,3, 4, 5 and RAS (HRAS, KRAS, or NRAS) mutations are found in about 50% of FTC and can also been found in benign adenomas.6, 7
In the earlier studies, several transgenic mouse models were created to study the molecular mechanisms of RAS-mediated transformation of thyroid follicular cells in vivo.8, 9, 10 Both PTC and FTC were developed from overexpression of a mutant RAS gene under TG promoter, which may not reflect the activity of endogenous mutant RAS gene expressed at the physiological level. Overexpression of KRAS and HRAS could also downregulate expression in a dose-dependent manner of NKX2-1, TG, and TPO genes required for thyroid hormone synthesis.11, 12 Recent knock-in mouse studies have shown that endogenous expression of either HRASG12V or KRASG12D is not sufficient to induce thyroid dysfunction and cell transformation under their native promoters.13, 14
It is known that TSH stimulates the growth or development of thyroid cancer and higher serum TSH is associated with both thyroid cancer incidence and recurrence.15, 16 It is not clear whether long-term TSH stimulation can induce KRASG12D-mediated thyroid follicular cell transformation. In the present study, we investigated the potential of long-term TSH stimulation on thyroid cancer development in KRASG12D knock-in mice targeted to express its oncoprotein in thyroid at the physiological level. We also studied the role of SPRY1 in the regulation of KRASG12D- vs BRAFV600E-mediated signaling.
MATERIALS AND METHODS
Experimental Animals
LSL-KRASG12D (obtained from The Jackson Laboratory, ME, USA), LSL-BRAFV600E, and TPO-Cre strains have been described previously.17, 18, 19 LSL-KRASG12D and LSL-BRAFV600E carry a latent mutant allele of KRAS and BRAF, respectively. Both LSL-KRASG12D and LSL-BRAFV600E mice were kept as heterozygotes. LSL-KRASG12D or LSL-BRAFV600E was crossed with TPO-Cre to generate TPO-KRASG12D or TPO-BRAFV600E strain where KRASG12D or BRAFV600E is conditionally expressed in thyroid follicular cells through Cre-mediated deletion of a floxed transcriptional stop sequence. The resulting TPO-KRASG12D or TPO-BRAFV600E strain expressed mutant KRASG12D or BRAFV600E transcripts at the physiological level under its endogenous promoter. The study was approved by the Animal Care and Usage Committee of the institution and conducted in compliance with the Public Health Service Guidelines for the Care and Use of Animals in Research.
Genotyping of TPO-KRASG12D or TPO-BRAFV600E Strain
Genotyping of TPO-Cre mediated recombination of LSL-KRASG12D or LSL-BRAFV600E targeted allele has been described previously.17, 19 Briefly, the following primers were used to detect LSL-KRASG12D recombination in mouse tissue: primer 1, 5′-GTCTTTCCCCAGCACAGTGC-3′, primer 2, CTCTTGCCTACGCCACCAGCTC-3′, and primer 3, 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′. Primer 1+2 detects wild-type allele yielding a product of 622 bp. Primer 1+2 also detects Cre-recombined KRASG12D allele (Lox-KRASG12D) yielding a product of 650 bp. This product is larger than the wild-type allele due to the presence of LoxP site that remains after Cre-mediated recombination. Primer 2+3 detects the LSL-KRASG12D allele yielding a product of 500 bp. Multiplex PCR containing three primers was used: 95 °C for 10 min followed by 35 cycles of amplification (95 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min with final extension at 72 °C for 10 min). The following primers were used to detect LSL-BRAFV600E recombination: primer A, 5′-AGTCAATCATCCACAGAGACCT-3′, primer B: 5′-GCTTGGCTGGACGTAAACTC-3′, and primer C, 5′-GCCCAGGCTCTTTATGAGAA-3′. Primer A+C detects the wild-type allele yielding a product of 466 bp. Primer A+C also detects Cre-recombined allele (Lox-BRAFV600E ) yielding a product of 518 bp. Primer B+C detects the LSL-BRAFV600E allele yielding a product of 140 bp. The PCR conditions are at 95 °C for 5 min followed by 35 cycles of amplification (95 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min with a final extension at 72 °C for 10 min).
Anti-Thyroid Drug Treatment
TPO-KRASG12D mice (4–10 weeks of age) were divided into two groups and given 0.1% (w/v) anti-thyroid drug propylthiouracil (PTU, Sigma-Aldrich, MO) in drinking water ad libitum, changed once weekly, to induce serum TSH. One group (n=10) was observed for 8 months and the other group (n=10) was observed for 14 months. Twenty TPO-KRASG12D mice were also divided into two groups (10 in each group) without PTU treatment, and observed for 8 and 14 months, respectively. Forty wild-type mice (TPO-KRASWT, 10 in each group) were given the same treatment as TPO-KRASG12D mice.
Thyroid Hormone Measurements
Blood was collected by cardiac puncture. Serum TSH was measured using MILLIPLEX MAP Mouse Pituitary Magnetic Bead Panel following the manufacturer’s instruction (EMD Millipore Corporation, Billerica, MA, USA). Serum total T4 was measured using MILLIPLEX MAP Steroid/Thyroid Hormone Magnetic Bead Panel (EMD Millipore Corporation).
Tumor Cell Culture
A PTC tumor from a 4-month-old TPO-BRAFV600E mouse was collected aseptically using blunt dissection and mechanically dissociated by mincing and passaged through a 40-μM mesh sterile screen, and suspended in DMEM/F12 growth medium (10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin). Cells were further dissociated by incubation in the growth medium containing 100 U/ml type I collagenase (Sigma-Aldrich) and 1.0 U/ml dispase I (Roche Diagnostics, Indianapolis, IN, USA) at 37 °C rocking water bath for 60 min. The cell suspension was washed twice and resuspended in 10 mm culture dish with DMEM/F12 growth medium containing 2 mU/ml bovine TSH (Sigma-Aldrich) to establish a BVE cell line (BRAFV600E-induced tumor cell line). The genetic background was confirmed by genotyping.
Quantitative Real-Time Reverse Transcriptase-PCR Analysis for SPRY1 Expression
Total RNA was isolated from thyroid of TPO-KRASWTmice treated with PTU, PTC of TPO-BRAFV600E, or FTC of TPO-KRASG12D mice by the quanidinium thiocyanate-phenol-chloroform method as described previously.20 The integrity of RNA was verified by denaturing gel electrophoresis. In all, 2 μg of each total RNA was reverse-transcribed to cDNA using the Promega RT system (Promega, Madison, WI, USA). LightCycler DNA Master SYBR Green 1 kit was used for quantitative real-time PCR analysis.21 The cDNA mix was diluted 10-fold, and 2 μl of the dilution was used for real-time PCR. PCR primers for the 114-bp SPRY1 cDNA fragment are 5′-GCGGAGGCCGAGGATTT-3′ (forward) and 5′-ATCACCACTAGCGAAGTGTGGC-3′ (reverse). The forward primer spans the junction of exon 1 and exon 2 over 1.7 kb intron 1 so that contaminated genomic DNA will not be amplified. The SPRY1 cDNA fragment was verified by DNA sequencing. The mRNA level of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, and a 155-bp PCR product was amplified using the following two primers: 5′-ATGTTCCAGTATGACTCCACTCACG-3′ (forward) and 5′-GAAGACACCAGTAGACTCCACGACA-3′ (reverse). The PCR conditions are 95 °C for 30 s followed by 30 cycles of amplification (95 °C for 10 s, 50 °C for 5 s, and 72 °C for 10 s). The resulting concentration of SPRY1 PCR products was normalized by comparison with GAPDH and was used to determine the relative mRNA level of SPRY1 in thyroid tumors.
Histology and Immunohistochemistry
Histology and immunohistochemical staining was described previously.22 Briefly, 4-μm-thick formalin-fixed paraffin-embedded tissue sections were prepared and stained with hematoxylin and eosin or SPRY1 (1:50 dilution, Abcam, Cambridge, MA, USA), p-ERK, or p-AKT antibody (1:50 dilution, Cell Signaling Technology, Danvers, MA, USA). Histological diagnosis was performed by a thyroid pathologist (MA) blinded to the genotype and the treatment status of the animal. DAKO LSAB+kit, HRP was used for immunostaining (DAKO, Carpinteria, CA, USA). The sections were counterstained with Mayer's haematoxylin.
Regulation of TSH Signaling by SPRY1
Mouse SPRY1 cDNA in pCMV6 expression vector was obtained from OriGene (Rockville, MD, USA). BVE cell line was transfected with either pCMV6 vector or mSPRY1/pCMV6 using Lipofectamine (Invitrogen, CA). The culture medium was changed to DMEM/F12 growth medium 16 h after transfection, and the transfected cells were cultured for additional 48 h in the presence or absence of 10 mU/ml bTSH. The expression of SPRY1, phospho-ERK, and phospho-AKT was determined by western blot analysis.
Western Blot Analysis
BVE cell line was transfected with mouse SPRY1 cDNA under the control of CMV promoter (Origen). 40 μg of protein was loaded onto a 12% SDS-polyacrylamide gel. Proteins were transferred onto a PVDF membrane and subject to western blot analysis using anti-phospho-ERK 1/2, and phosphor-AKT antibody (1:1000, Cell Signaling Technology) or anti-SPRY1 (1:1000, Abcam).
Bioinfomatic Anylysis of Human PTC Samples
The expression profiles of SPRY1, SPRY2, SPRY3, and SPRY4 in the TCGA data set of 572 PTC samples23 were analyzed using UCSC cancer genomics browser (https://genome-cancer.soe.ucsc.edu/proj/site/hgHeatmap/).
Statistical Analysis
Unpaired Student’s t-test (two-tailed) was used. A P-value of 0.05 or less was considered as significant.
RESULTS
Without PTU treatment, only mild thyroid enlargement and hyperplasia were observed in TPO-KRASG12D mice (Figure 1b). The thyroids were about two times larger as compared with those from the TPO-KRASWT mice. Thyroid cancer development was not observed among 10 mice monitored for up to 14 months. With PTU treatment, significant thyroid enlargement with multinodular goiters and hyperplasia occurred in both TPO-KRASG12D and TPO-KRASWTmice (Figure 2a). Massive thyroid enlargement was found in TPO-KRASG12D mice: thyroids of TPO-KRASG12D mice were about six times larger than TPO-KRASWT mice (118.75±13.15 mg vs 21.11±6.33 mg, P<0.001, Figure 2b), indicating that TSH can cooperate with KRASG12D to promote thyroid growth and hyperplasia. Interestingly, thyroid histology of TPO-KRASG12D mice showed nodular hyperplasia with well-maintained follicular architecture whereas in TPO-KRASWT mice such follicular architecture was replaced by papillary hyperplasia (Figure 2c). However, PTC was not diagnosed due to lack of characteristic nuclear features such as nuclear grooves, intranuclear cytoplasmic inclusion, fine chromatin texture, and nuclear envelope irregularity. As shown in Figure 2d, serum TSH was significantly elevated in PTU-treated mice as compared with the control (24 370.8±12 942.5 vs 426.4±9.65 pg/ml, P<0.01). Serum T4 was significantly reduced in PTU-treated mice as compared with the control (5.21±1.31 vs 22.68±6.23 ng/ml, P<0.05). Among 10 TPO-KRASG12D mice monitored for 14 months, two developed follicular thyroid cancer (FTC, Figure 3a) and one with pulmonary metastasis (Figure 3b). Since the cellular features were indistinguishable between follicular hyperplasia and cancer, the diagnosis of FTC was based on the invasion of surrounding tissues such as blood vessel and neck muscle (Figure 3a), and pulmonary metastasis (Figure 3b). Among 10 TPO-KRASWT mice monitored for 14 months under PTU treatment, no tumor development was observed.
Activation of KRASG12D in thyroid induces mild thyroid enlargement and hyperplasia. (a) Genotyping of TPO-Cre mediated recombination of LSL-KRAS G12D (A) and LSL-BRAFV600E (B). The PCR product was run on a 1.5% agarose gel. The activation of KRASG12D and BRAFV600E occurred only in the thyroid as a result of Cre-mediated deletion of a floxed transcriptional stop sequence. (b) Mild thyroid enlargement and hyperplasia were observed in TPO-KRASG12D mice. Thyroids of a TPO-KRASWT mouse (A) and a TPO-KRASG12D mouse (B) at the age of 8 months were shown at the top panel. Histology of thyroids from the same TPO-KRASWT mice (C, × 20) and TPO-KRASG12D mice (D, × 20) was shown at the bottom panel.
TSH cooperates with KRASG12D to induce massive thyroid hyperplasia. (a) Thyroids of a TPO-KRASwt mouse (A) and a TPO-KRASG12D mouse (B) at 16 months old were shown. The mice were treated with PTU in drinking water for 14 months. Goiters were present in both mice and significant thyroid enlargement was observed in TPO-KRASG12D mice. (b) Thyroid weight in PTU-treated mice. Thyroid was collected from 10 TPO-KRASG12D and 10 TPO-KRASWT mice treated with PTU for 14 months (at the end of experiment). Thyroid weight was measured and data were expressed as mean±s.e.m., *P<0.001. (c) Histology of thyroids from the same TPO-KRASWT and TPO-KRASG12D mice was shown. Follicular architecture was replaced by papillary structure in hyperplastic goiters from a TPO-KRASWT mouse (A, × 4; C, × 20) whereas in the goiter of a TPO-KRASG12D mouse, the follicular architecture was intact (B, × 4, D, × 20). (d) Thyroid hormone levels in PTU-treated mice. Serum was collected from six TPO-KRAS mice treated with PTU for 1 month and from six mice of same age group without PTU treatment. The samples were assayed individually in triplicates. The data are expressed as mean±s.em., *P<0.05.
TSH cooperates with KRASG12D to induce transformation of thyroid follicular cells into FTC. (a) Thyroid of a TPO-KRASG12D mouse with FTC following 14-month PTU treatment (A). FTC with neck muscle invasion as indicated by arrows (B, × 10) and blood vessel invasion (indicated by arrows, C, × 20; D, × 40). (b) Pulmonary metastasis of FTC from the same mice. Multiple foci of metastasis can be seen from gross examination of the lung (A) and microscopic examination (B, × 10; C, × 20, D, × 40).
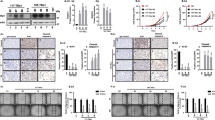
The Sprouty (SPRY) family of proteins is involved in the negative feedback regulation of growth factor-mediated MAPK activation.24 To understand why TSH stimulation caused only FTC, but not PTC, we investigated SPRY1 expression in thyroid tumors from TPO-KRASG12D and TPO-BRAFV600E mice. SPRY1 mRNA level was significantly higher in KRASG12D-induced FTC (9.23±0.56) as compared with KRASWTcontrol (1.03±0.15, P<0.01, Figure 4a). SPRY1 expression was also increased in KRASG12D thyroid without PTU treatment (2.1±0.2, P<0.01, Figure 4a), but the expression level was much lower than under PTU treatment, suggesting that the increased SPRY1 expression may be a compensatory mechanism to counter TSH-mediated growth stimulation. In contrast, SPRY1 mRNA level was significantly lower in BRAFV600E-induced PTC (0.31±0.17) as compared with BRAFWT control (1.00±0.12, P<0.05, Figure 4b). The serum TSH was greater than 50 000 pg/ml (beyond the detection limit of the kit) and T4 was 3.87±0.68 in TPO-BRAFV600E mice (n=6) as compared with 445.2±72.21 in TSH and 29.03±3.62 in T4 from TPO-BRAFWT mice (n=6), consistent with previous report that TPO-BRAFV600E mice have severe hypothyroidism.13 The increased SPRY1 expression in FTC was confirmed by immunohistochemistry analysis. As shown in Figure 4c, increased SPRY1 and p-AKT expression was observed in FTC (b and e) with normal p-ERK expression (h). In PTC, both p-ERK and p-AKT expression were increased (f and i). Although downregulation of SPRY1 expression in PTC was not clearly shown by immunohistochemistry (Figure 4cC), it was confirmed by western blot analysis, showing inverse correlation between SPRY1 expression and p-ERK activation (Figure 4d).
SPRY1 expression in thyroid cancer. SPRY1 mRNA from KRASG12D-induced FTC (a) and BRAFV600E-induced PTC (b) was analyzed by qRT-PCR. RNA from TPO-KRASWT mouse thyroids and TPO-BRAFWTmouse thyroids was used as a control. Data were expressed as mean±s.e.m. of three separate experiments. (c) Immunohistochemistry staining of SPRY1, p-AKT, and p-ERK. Normal thyroid (A, D, and G, × 40), FTC from a TPO-KRASG12D mouse (B, E, and H, × 40), and PTC from a TPO-BRAFV600E mouse were stained with SPRY1 antibody (A, B, and C, 1:50 dilution), p-AKT antibody (D, E, and F, 1:50 dilution) and p-ERK antibody (G, H, and I, 1:50 dilution). Increased expression of SPRY1 and p-AKT was seen in FTC. p-ERK was not activated in FTC whereas both p-AKT and p-ERK were activated in PTC. (d) Western blot analysis of SPRY1 expression in a PTC tumor. Decreased SPRY1 expression is observed in PTC as compared with normal thyroid.
To further investigate the role of SPRY1 in the regulation of TSH-mediated signaling, we used BVE cell line (derived from BRAFV600E-induced PTC tumor) as a model, for it has low SPRY1 expression. The cell line was transfected with mouse SPRY1 cDNA and treated with or without TSH. As shown in Figure 5, SPRY1 expression reduced p-ERK expression, and TSH stimulation only activated p-ATK when SPRY1 expression was increased. When SPRY1 expression was reduced, TSH stimulation increased both p-ERK and p-ATK expression (Figure 5). These data suggest that SPRY1 may act as a molecular switch to control thyroid follicular cells to go through either MAP kinase (RAF-MEK-ERK) or PI3 kinase (PI3K/AKT) signaling pathway. When SPRY1 is upregulated by KRASG12D, MAPK pathway is inhibited and only PI3K/AKT pathway is utilized, resulting in FTC. When SPRY1 is downregulated by BRAFV600E, thyroid follicular cells may go through both MAPK and PI3K/AKT pathways to initiate PTC.
Regulation of TSH signaling by SPRY1. BVE cell line was transfected with mouse SPRY1 cDNA and cultured in the presence or absence of 10 mU/ml bovine TSH for 48 h. The expression of SPRY1, phospho-ERK, and phospho-AKT was determined by western blot analysis. Reduction of p-ERK is observed by increased SPRY1 expression, and TSH stimulation only activates p-ATK in the presence of increased SPRY1 expression. TSH can increase both p-ERK and p-ATK expression when SPRY1 expression is reduced.
Finally, we performed bioinformatic mining of the recent TCGA data set of 572 human PTC samples to compare SPRY gene (SPRY1, SPRY2, SPRY3, and SPRY4) expression between classic PTC (CPTC) and follicular variant PTC (FVPTC). FVPTC shares many molecular and pathological features with FTC. RAS mutation is frequently detected in FVPTC as compared with frequent BRAF mutation in CPTC. As shown in Figure 6, the SPRY1 and SPRY4 expression is reduced in normal thyroids. The expression of SPRY2 and SPRY3 tends to increase in normal thyroids and is variable in both CPTC and FVPTC. The SPRY1 expression is also variable in CPTC and FVPTC. In CPTC, increased SPRY1 expression is present in 36.6% (162/409) samples, decreased expression in 40.6% (166/409) and unchanged in 19.8% (81/409) samples. In FVPTC, 40.6% (43/106) samples have increased expression, 36.8% (39/106) have decreased expression, and 22.6% (24/106) unchanged. The expression of SPRY4 is consistently increased in FVPTC: 66.1% (72/109) have increased expression and 13.8% (15/109) have decreased expression. These data suggest that the SPRY family of proteins is involved in the regulation of thyroid tumorigenesis. The exact role of each protein remains to be determined.
The gene expression profile of SPRY family of proteins in human thyroid cancer. The TCGA thyroid cancer gene expression data set of 572 thyroid cancer samples was used to examine gene expression profile among normal thyroid, PTC, classic PTC (CPTC), tall cell PTC, and follicular variant PTC (FVPTC). The gene expression profile is presented as a heatmap of red (upregulated), black (no change), and green (downregulated). The SPRY1 and SPRY4 expression is downregulated in normal thyroids. The expression of SPRY4 is consistently increased in FVPTC.
DISCUSSION
In the present study, we have demonstrated that KRASG12D mutation alone is not sufficient to induce oncogenic transformation of thyroid follicular cells. With long-term TSH stimulation, KRASG12D can promote thyroid growth and hyperplasia, and eventually result in oncogenic transformation into follicular thyroid cancer. This demonstrates the significant oncogenic role of TSH in thyroid tumorigenesis: without TSH stimulation, thyroid cancer would not be able to develop or progress even in the presence of KRASG12D or BRAFV600E.25
The animal model in the current study is very similar to congenital hypothyroidism in humans, which is caused by defects in thyroid hormone synthesis.26 High levels of TSH are often present in patients with congenital hypothyroidism due to poor management or patients’ non-compliance to treatment. We and others have reported thyroid cancer development as a result of long-term TSH stimulation.27, 28 Although both PTC and FTC were reported in those cases, BRAF mutation was found only in patients with PTC.29 RAS mutations have been reported in both benign thyroid goiters and cancers,30 indicating that additional factors are required to initiate malignant transformation. The current study has demonstrated that chronic TSH stimulation is one of the factors required for RAS-mediated carcinogenesis.
TSH stimulates growth and differentiation of thyroid follicular cells through its G protein-coupled receptor. TSH can activate both cAMP-protein kinase A (PKA) pathway for differentiation31 and RAS-PI3K pathway for proliferation.32, 33 RAS allows growth promoting signals through both MAPK (RAF-MEK-ERK) and PIK3CA/AKT pathways. Previous studies have shown that cAMP can inhibit RAS-mediated signals to MAPK by blocking RAF-1 activation via RAP1.34, 35 Under these circumstances, PI3K signaling is the main mediator of RAS effects. RAP1 is a member of the RAS family of small G proteins that transmit signals from cAMP-PKA pathway to MAPK pathway by interaction with BRAF.36 Without BRAF, GTP-loaded RAP1 blocks RAS activation of RAF-1, thereby inhibiting MAPK signaling.37 In a mouse model with constitutive RAP1 activation in thyroid, FTC was not developed until given 12 months goitrogen treatment, indicating that TSH signaling is required and is probably via RAS-PI3K pathway for FTC initiation.38 In two different FTC mouse models, TSH signaling is constantly activated with simultaneous activation of either PI3K/AKT39, 40 or phospholipase C pathway.41 FTC was developed in all mice with PI3K/AKT activation, suggesting that PI3K is an important mediator of RAS effects in TSH-induced FTC. FTC can develop rapidly without TSH signaling when both RAS and PI3K/AKT pathways are constitutively activated.42 Although MAPK is activated in these mice,42 PI3K signaling probably predominates over MAPK, resulting in FTC phenotype. Since serum TSH is very low in these mice, MAPK activation may reflect the loss of cAMP-mediated inhibition of RAS signals to MAPK.43
TSH signaling promotes the tumorigenesis of PTC,13 indicating a cross-talk between TSH-cAMP-PKA pathway and MAPK pathway.37 As discussed above, RAP1 may be one of the molecular switches to control TSH-cAMP signaling through either RAS-PI3K to initiate FTC or RAS-MAPK to induce PTC. In the current study, we have identified that SPRY1 is another molecular switch to regulate RAS-MAPK signaling. SPRY1 has been reported as a candidate tumor-suppressor gene in medullary thyroid carcinoma44 and its expression is decreased in human prostate cancer.45 We also found reduced SPRY1 expression in human PTC with BRAFV600E (data will be presented elsewhere). Concomitant loss of SPRY1 and SPRY2 results in hyperactive MAPK signaling and low-grade prostatic intraepithelial neoplasia.46 However, when SPRY1 and SPRY2 loss-of-function occurs in the context of loss of one Pten allele, aberrant activation of AKT and invasive neoplasia occur, suggesting that Sprouty genes can negatively regulate the PI3K/AKT pathway as well.46 Indeed, PI3K/AKT activation has been reported in PTC patients with BRAFV600E.47 Our data also demonstrate the activation of both MAPK and PI3K/AKT pathways in BRAFV600E-induced PTC when SPRY1 expression is reduced. In KRASG12D-induced lung cancer, SPRY2 is upregulated via negative feedback loop to inhibit MAPK signaling.48 However, we only found SPRY1 upregulation in KRASG12D-induced FTC and SPRY2 expression was not changed (data not shown). The differential SPRY1 expression in TPO-KRASG12D and TPO-BRAFV600E mice indicates that SPRY1 can selectively regulate TSH-mediated RAS signaling pathways in thyroid: favoring MAPK pathway and PTC initiation when its expression is downregulated and PI3K/AKT pathway and FTC initiation when its expression is upregulated. It has been reported that supraphysiologic expression of a mutant RAS can result in PTC or mixed papillary–follicular features in early transgenic mice studies,8, 10 which appears to be contradictory to our hypothesis. However, overexpressed mutant RAS beyond physiological level may escape SPRY1-mediated feedback inhibition of MAPK signaling and induce PTC.
References
Davies L, Welch HG . Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164–2167.
Hundahl SA, Fleming ID, Fremgen AM et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer 1998;83:2638–2648.
Kimura ET, Nikiforova MN, Zhu Z et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003;63:1454–1457.
Cohen Y, Xing M, Mambo E et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625–627.
Xing M . BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12:245–262.
Vasko V, Ferrand M, Di Cristofaro J et al. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab 2003;88:2745–2752.
Fukahori M, Yoshida A, Hayashi H et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid 2012;22:683–689.
Rochefort P, Caillou B, Michiels FM et al. Thyroid pathologies in transgenic mice expressing a human activated Ras gene driven by a thyroglobulin promoter. Oncogene 1996;12:111–118.
Santelli G, de Franciscis V, Portella G et al. Production of transgenic mice expressing the Ki-ras oncogene under the control of a thyroglobulin promoter. Cancer Res 1993;53:5523–5527.
Vitagliano D, Portella G, Troncone G et al. Thyroid targeting of the N-ras(Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene 2006;25:5467–5474.
Francis-Lang H, Zannini M, De Felice M et al. Multiple mechanisms of interference between transformation and differentiation in thyroid cells. Mol Cell Biol 1992;12:5793–5800.
De Vita G, Bauer L, da Costa VM et al. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol 2005;19:76–89.
Franco AT, Malaguarnera R, Refetoff S et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA 2011;108:1615–1620.
Charles RP, Iezza G, Amendola E et al. Mutationally activated BRAF(V600E) elicits papillary thyroid cancer in the adult mouse. Cancer Res 2011;71:3863–3871.
Kim HK, Yoon JH, Kim SJ et al. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–477.
Boelaert K . The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009;16:1065–1072.
Jackson EL, Willis N, Mercer K et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001;15:3243–3248.
Kusakabe T, Kawaguchi A, Kawaguchi R et al. Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis 2004;39:212–216.
Mercer K, Giblett S, Green S et al. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res 2005;65:11493–11500.
Zou M, Baitei EY, Alzahrani AS et al. Oncogenic activation of MAP kinase by BRAF pseudogene in thyroid tumors. Neoplasia 2009;11:57–65.
Zou M, Famulski KS, Parhar RS et al. Microarray analysis of metastasis-associated gene expression profiling in a murine model of thyroid carcinoma pulmonary metastasis: identification of S100A4 (Mts1) gene overexpression as a poor prognostic marker for thyroid carcinoma. J Clin Endocrinol Metab 2004;89:6146–6154.
Zou M, Al-Baradie RS, Al-Hindi H et al. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer 2005;93:1277–1284.
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–690.
Hanafusa H, Torii S, Yasunaga T et al. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 2002;4:850–858.
Shimamura M, Nakahara M, Orim F et al. Postnatal expression of BRAFV600E does not induce thyroid cancer in mouse models of thyroid papillary carcinoma. Endocrinology 2013;154:4423–4430.
Kopp P . Perspective: genetic defects in the etiology of congenital hypothyroidism. Endocrinology 2002;143:2019–2024.
Alzahrani AS, Baitei EY, Zou M et al. Clinical case seminar: metastatic follicular thyroid carcinoma arising from congenital goiter as a result of a novel splice donor site mutation in the thyroglobulin gene. J Clin Endocrinol Metab 2006;91:740–746.
Raef H, Al-Rijjal R, Al-Shehri S et al. Biallelic p.R2223H mutation in the thyroglobulin gene causes thyroglobulin retention and severe hypothyroidism with subsequent development of thyroid carcinoma. J Clin Endocrinol Metab 2010;95:1000–1006.
Hishinuma A, Fukata S, Kakudo K et al. High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid 2005;15:1079–1084.
Shi YF, Zou MJ, Schmidt H et al. High rates of ras codon 61 mutation in thyroid tumors in an iodide-deficient area. Cancer Res 1991;51:2690–2693.
Laugwitz KL, Allgeier A, Offermanns S et al. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA 1996;93:116–120.
Ciullo I, Diez-Roux G, Di Domenico M et al. cAMP signaling selectively influences Ras effectors pathways. Oncogene 2001;20:1186–1192.
Suh JM, Song JH, Kim DW et al. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem 2003;278:21960–21971.
Cook SJ, McCormick F . Inhibition by cAMP of Ras-dependent activation of Raf. Science 1993;262:1069–1072.
Schmitt JM, Stork PJ . Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol Cell Biol 2001;21:3671–3683.
Wang Z, Dillon TJ, Pokala V et al. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol 2006;26:2130–2145.
Stork PJ, Schmitt JM . Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 2002;12:258–266.
Ribeiro-Neto F, Leon A, Urbani-Brocard J et al. cAMP-dependent oncogenic action of Rap1b in the thyroid gland. J Biol Chem 2004;279:46868–46875.
Suzuki H, Willingham MC, Cheng SY . Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 2002;12:963–969.
Furuya F, Lu C, Willingham MC et al. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 2007;28:2451–2458.
Ledent C, Denef JF, Cottecchia S et al. Costimulation of adenylyl cyclase and phospholipase C by a mutant alpha 1B-adrenergic receptor transgene promotes malignant transformation of thyroid follicular cells. Endocrinology 1997;138:369–378.
Miller KA, Yeager N, Baker K et al. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res 2009;69:3689–3694.
Li Y, Takahashi M, Stork PJ . Ras-mutant cancer cells display B-Raf binding to Ras that activates extracellular signal-regulated kinase and is inhibited by protein kinase A phosphorylation. J Biol Chem 2013;288:27646–27657.
Macia A, Gallel P, Vaquero M et al. Sprouty1 is a candidate tumor-suppressor gene in medullary thyroid carcinoma. Oncogene 2012;31:3961–3972.
Kwabi-Addo B, Wang J, Erdem H et al. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res 2004;64:4728–4735.
Schutzman JL, Martin GR . Sprouty genes function in suppression of prostate tumorigenesis. Proc Natl Acad Sci USA 2012;109:20023–20028.
Faustino A, Couto JP, Populo H et al. mTOR pathway overactivation in BRAF mutated papillary thyroid carcinoma. J Clin Endocrinol Metab 2012;97:E1139–E1149.
Shaw AT, Meissner A, Dowdle JA et al. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev 2007;21:694–707.
Acknowledgements
The study is supported by KACST grants (11-BIO1434-20 and PL-10-0051). We would like to thank Dr Mario Encinas for critical discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This paper investigates the potential of long-term exposure to thyroid stimulating hormone (TSH) and expression of the negative regulator of receptor tyrosine kinase signaling, SPRY1, on thyroid cancer development. SPRY1 expression is increased in follicular thyroid cancer and promotes TSH-RAS signaling through the PI3K/AKT pathway. However, SPRY1 is down-regulated in papillary thyroid cancer and results in both MAPK and PI3K/AKT activation.
Rights and permissions
About this article
Cite this article
Zou, M., Baitei, E., Al-Rijjal, R. et al. KRASG12D-mediated oncogenic transformation of thyroid follicular cells requires long-term TSH stimulation and is regulated by SPRY1. Lab Invest 95, 1269–1277 (2015). https://doi.org/10.1038/labinvest.2015.90
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.90