Abstract
Integrin α6β4 is a cellular adhesion molecule that binds to laminins in the extracellular matrix and nucleates the formation of hemidesmosomes. During carcinoma progression, integrin α6β4 is released from hemidesmosomes, where it can then signal to facilitate multiple aspects of tumor progression including sustaining proliferative signaling, tumor invasion and metastasis, evasion of apoptosis, and stimulation of angiogenesis. The integrin achieves these ends by cooperating with growth factor receptors including EGFR, ErbB-2, and c-Met to amplify downstream pathways such as PI3K, AKT, MAPK, and the Rho family small GTPases. Furthermore, it dramatically alters the transcriptome toward a more invasive phenotype by controlling promoter DNA demethylation of invasion and metastasis-associated proteins, such as S100A4 and autotaxin, and upregulates and activates key tumor-promoting transcription factors such as the NFATs and NF-κB. Expression of integrin α6β4 has been studied in many human malignancies where its overexpression is associated with aggressive behavior and a poor prognosis. This review provides an assessment of integrin α6β4 expression patterns and their prognostic significance in human malignancies, and describes key signaling functions of integrin α6β4 that contribute to tumor progression.
Similar content being viewed by others
Main
Integrins are cellular adhesion molecules that serve as receptors for extracellular matrix (ECM) components and select cell adhesion molecules. Named for their ability to integrate signals from the extracellular environment to the inside of the cell, integrins are responsible for securing cells to the surrounding adhesion molecules while amplifying and potentiating signals from growth factor receptors and other extracellular stimuli.1 These transmembrane proteins permit cells to sense and respond to their environment and thus play critical roles in maintaining normal cellular functions, yet have also been implicated in promoting invasion and metastasis in human malignancies.2
Integrins are heterodimeric receptors that consist of paired α and β subunits. In the human genome, there are 18 α and 8 β subunits that combine in a limited combination to provide 24 integrin receptors, each with its own specificity for select ECM or cellular adhesion proteins (for review, see Hynes1 and Barczyk et al3). Integrins containing the α6 subunit are laminin receptors in which the α6 subunit can pair with either the β1 or β4 subunit. In contrast, the integrin β4 subunit can only pair with the α6 subunit,1, 2, 4 thus making β4 subunit expression predictive of integrin α6β4 expression.
Integrin α6β4 is predominantly expressed in epithelial cells where it is present at the basal surface adjacent to the basement membrane and nucleates the formation of hemidesmosomes. These stable adhesions are critical for the integrity of epithelial monolayers. In contrast to this function, integrin α6β4 signaling in various cancers promotes an invasive and metastatic phenotype. This functional change is mediated by phosphorylation of the cytoplasmic tail of the integrin β4 subunit that releases integrin α6β4 from hemidesmosomes and allows the integrin to promote invasive signaling through cooperation with growth factor receptors and alteration of the transcriptome that in turn facilitates tumor progression.2, 4, 5, 6, 7, 8, 9 Below, we highlight key signaling functions of integrin α6β4 and provide a review of its expression patterns and their prognostic significance in human malignancies.
STRUCTURE AND NORMAL FUNCTION OF INTEGRIN α6β4
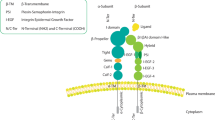
The α6β4 integrin is a specialized integrin that is expressed in various normal epithelia, Schwann cells, and endothelial cells. The integrin β4 subunit is distinct from other integrin subunits in that it has a particularly long cytoplasmic signaling domain. Whereas the cytoplasmic domains of other integrin subunits are <50 amino acids in length, the integrin β4 subunit is >1000 amino acids in length.10, 11 As depicted in Figure 1, the cytoplasmic domain of the integrin β4 subunit is characterized by two pairs of fibronectin type III domains, a Calxβ domain and a connecting segment.12 The fibronectin repeats and the connecting segment are necessary for hemidesmosome assembly.13, 14, 15
Integrin α6β4 structure and hemidesmosome assembly. (a) The integrin β4 subunit only pairs with the α6 subunit. The long cytoplasmic domain of β4 is structurally distinct from other known receptors but contains several distinct domains including a Calx-β domain, four fibronectin type III repeats, a connecting segment, and C-terminal tail. (b) Integrin α6β4 nucleates hemidesmosomes by binding to multiple hemidesmosomal-associated proteins including Plectin (HD1), BP180 (also known as BPAG2 or collagen XVII), and BP230 (also known as BPAG1).
At the basal surface of normal cells adjacent to the basement membrane, integrin α6β4 binds to laminins in the ECM and facilitates stable adhesion through the formation of hemidesmosomes. Hemidesmosomes are large adhesion complexes that anchor the basal layer of epithelial cells to the basement membrane.13, 14, 15 In these junctions, the α6β4 integrin nucleates the connection between cytokeratin intermediate filaments in the cell and laminins in the basement membrane through its interactions with plectin, collagen XVII/BP180, and BP230, as depicted in Figure 1b.16, 17 The importance of these junctions is highlighted by the fact that mutations in the integrin β4 (ITGB4) gene can cause lethal forms of epidermolysis bullosa with pyloric atresia, a disorder characterized by blistering and ulceration of the skin and mucosal tissues.18 Although it has been suggested that other integrins may be able to compensate for a loss of integrin α6β4, studies in mouse models have demonstrated that this is not the case.19 Integrin β4 knockout mice (−/−) are born with severe epidermal blistering, exhibit widespread separation of the epithelial–mesenchymal junction, and die shortly after birth. These observations emphasize the importance of the integrin β4 subunit in maintaining the integrity of the epithelial–ECM junction.19
Hemidesmosomes are dynamic structures. During wound healing, hemidesmosomes must be dismantled to allow the leading edge cells to migrate into the wound.14, 20 This functional change is mediated by phosphorylation of the cytoplasmic tail of the integrin β4 subunit that releases integrin α6β4 from hemidesmosomes. This process occurs through stimulation by growth factor receptors such as the epidermal growth factor receptor (EGFR), and by direct phosphorylation of the integrin β4 cytoplasmic tail by protein kinase C.7, 8, 21 Upon release from hemidesmosomes, integrin α6β4 relocalizes from the keratin cytoskeleton to the actin cytoskeleton.22 When bound to F-actin, the integrin α6β4 signaling domain promotes the formation of motility structures, such as filopodia and lamellae, by cooperating with growth factor receptors and stimulating key signaling pathways6, 7, 22, 23, 24 that in turn facilitate migration and wound closure.
Integrin α6β4 and hemidesmosomes are also suggested to play a role in the contextual orientation of cells. When cells are not adhered to the proper extracellular matrices, they will undergo a specialized form of apoptosis known as anoikis. Notably, when caspases are activated, the β4 cytoplasmic tail is cleaved leading to apoptosis.25, 26
INTEGRIN α6β4 SIGNALING IN MALIGNANT CELLS
Release of integrin α6β4 from hemidesmosomes can lead to altered signals that promote tumor cell growth, invasion, and metastasis.2, 4, 5, 6, 7, 8, 9 Under conditions where hemidesmosomes are disassembled, integrin α6β4 binding directly to laminin has been shown to activate both phosphoinositide 3-OH kinase (PI3K) and RhoA small GTPases.6, 24 Alternatively, the integrin can cooperate with multiple different growth factor receptors including those in the EGF receptor family (ErbB-1,2,3), c-Met, LPA1, LPA2, and Ron23, 27, 28, 29, 30 to enhance signaling through PI3K, AKT, MAPK, and the Rho small GTPases,6, 23, 24, 31, 32 as depicted in Figure 2. In cells with mutant p53, the α6β4 integrin promotes cell survival through activation of AKT/PKB31 and stimulates cell cycle progression and proliferation by interacting with Shc to activate the Ras/MAPK pathway.32 As shown in breast and pancreatic cancers, this integrin can promote tumor progression through transcriptional regulation and has been shown to increase the expression of invasive and metastatic proteins such as the epithelial to mesenchymal transition (EMT)-associated protein S100A4 (also known as metastasin/FSP).33, 34, 35 In the next several sections, we discuss our current understanding of how integrin α6β4 signaling promotes a malignant phenotype with an emphasis on its impact on invasion, cell survival, and angiogenesis.
Cancer progression-associated signaling pathways activated by integrin α6β4. Integrin α6β4 can activate multiple signal transduction cascades either directly by binding its ligand laminin or by amplifying signals from multiple growth factors. Enhanced signaling through these pathways contributes to tumor progression in terms of enhanced proliferation, cell survival, invasion, and metastasis. Notably, integrin α6β4 can act as a tumor suppressor by promoting apoptosis in the presence of a wild-type p53. For this reason, integrin α6β4 is often overexpressed primarily in tumor types where p53 mutations are prominent such as pancreatic and basal-like breast cancers.
Invasive Signaling Functions
The finding that integrin α6β4 mediates stable adhesive complexes that anchor cells to the basement membrane would seem to argue against the participation of integrin α6β4 in cell migration. Despite this apparent contradiction, numerous studies have confirmed that integrin α6β4 is responsible for promoting migratory and invasive behavior in carcinoma cells. A potential explanation for this phenomenon comes from its normal role in wound healing,14, 20, 36 as described above.
Early studies in colon and breast carcinoma lines demonstrated that expression of integrin α6β4 contributes to tumor cell invasiveness.6, 9, 37 These studies led to the pivotal discovery that integrin α6β4 can promote invasive properties in carcinoma cells by activating the PI3K pathway,6 a signaling pathway that is now well known for its role in promoting carcinoma progression.38 Notably, this was the first time that PI3K had been implicated in carcinoma invasion. This finding was subsequently confirmed in a number of additional reports,39, 40, 41 although the exact mechanism by which integrin α6β4 activates PI3K initially remained elusive. The cytoplasmic domain of the integrin β4 subunit does not contain a consensus-binding motif for the regulatory subunit of PI3K, making direct activation of this pathway by integrin β4 subunit unlikely.4, 6, 42 One mechanism for integrin β4-mediated activation of PI3K was found to involve insulin receptor substrate-1 and -2 (IRS-1 and IRS-2) that act as signaling intermediates that facilitate integrin α6β4-mediated PI3K activation.42 Ligation of integrin α6β4 promotes phosphorylation of IRS-1 and IRS-2, leading to subsequent activation of PI3K.42 An additional mechanism has been described wherein integrin α6β4 cooperates with ErbB-2 to promote PI3K-dependent invasion.39 Finally, integrin α6β4 has been shown to localize to lipid rafts in the plasma membrane that may allow it to activate PI3K by facilitating close interactions with other receptor tyrosine kinases.4, 40
The involvement of integrin α6β4 in cell polarization and the formation of F-actin-rich motility structures such as filopodia and lamellae lead to investigations into the Rho family of small GTPases. The Rho family of small GTPases largely control the reorganization of the actin cytoskeleton needed for cell motility.43 Initial studies by Shaw et al6 on the activation and function of PI3K in invasion found that the small GTPase Rac1 was required for invasion downstream of PI3K, a finding confirmed by others.44 Notably, integrin α6β4 can cooperate with growth factor receptors45 and other integrins23 to activate Rac1. Further studies examining the impact of integrin α6β4 on migration and invasion found that integrin α6β4 increased the activity of cAMP-specific phosphodiesterase, thereby resulting in decreased cAMP concentrations and subsequent RhoA activation.9, 24 The activation of RhoA downstream of integrin α6β4 subsequently leads to the formation of RhoA-dependent membrane ruffling and lamellae formation, as well as the generation of contraction forces that enable cell migration.24, 46 Our group found that the metastasis-associated protein S100A4, which is regulated by integrin α6β4,35 interacts with Rho effector Rhotekin to promote cell membrane ruffling.47 Notably, the traditional function for RhoA is the generation of stress fibers rather than membrane ruffling, suggesting that integrin α6β4 can change the function of RhoA to facilitate tumor invasion.
Integrin α6β4 amplifies signaling through multiple receptor tyrosine kinases and G protein-coupled receptors in order to promote tumor cell proliferation and invasion. Notably, cooperative signaling has been identified between integrin α6β4 and multiple members of the EGFR family. EGFR and integrin α6β4 have also been shown to colocalize at the leading edge of carcinoma cells subjected to EGF stimulation, and their interaction was inhibited by curcumin, a compound present in turmeric.48 Integrin α6β4 has also been shown to associate with ErbB-2 in multiple breast carcinoma cell lines,30, 49 although reports are conflicting as to whether integrin α6β4 expression correlates with ErbB-2 protein overexpression in patient-derived carcinoma tissues.50, 51, 52, 53 Using a mouse model of ErbB-2-mediated tumorigenesis, loss of integrin β4 signaling was shown to reduce breast tumor invasive growth and metastasis, and deletion of the integrin β4 signaling domain was shown to enhance the efficacy of ErbB2-targeted therapy. This study also demonstrated that the integrin β4 subunit forms a complex with ErbB-2 and amplifies ErbB-2 signaling.27 In addition, integrin α6β4 has been shown to regulate the expression of ErbB-2 by influencing its translation.54 These findings are particularly notable as ErbB-2 amplification promotes invasion in human malignancies such as breast carcinoma, and is associated with aggressive behavior.55, 56
Although an interaction with ErbB-2 may be necessary in order for integrin α6β4 to activate PI3K-mediated invasion in select cell types, ErbB-2 lacks a consensus-binding site for the regulatory subunit of PI3K. Furthermore, ErbB-2 must dimerize with an EGFR family member in order to function.30, 57 A potential solution to this issue was suggested by the finding that integrin α6β4-mediated PI3K activation is dependent on ErbB-2/ErbB-3 heterodimerization.30 The ErbB-2/ErbB-3 heterodimer is a strong activator of PI3K,30, 58 and the ErbB-3 cytoplasmic domain contains binding sites for the regulatory subunit of PI3K.59 Integrin α6β4 can regulate the expression of ErbB-3, leading to an increase in ErbB-2/ErbB-3 heterodimerization and subsequent PI3K activation,30 and notably, a positive association has been identified between integrin α6β4 and ErbB-3 expression in patient-derived tumors.51
Integrin α6β4 can also cooperate with c-Met, a receptor tyrosine kinase activated by the hepatocyte growth factor (HGF).28, 29 In one study, integrin α6β4 was shown to form a direct complex with c-Met that promotes HGF-dependent invasion.28 Additional studies have shown that although an interaction with integrin α6β4 may contribute to c-Met-dependent invasion, integrin α6β4 and c-Met are also able to promote invasion independently.29 Evidence for a physical association between integrin α6β4 and c-Met is controversial;4, 29 however, this does not preclude the probability that integrin α6β4 can cooperate with c-Met without physical association.
Ron (‘recepteur d'origine nantais’), a tyrosine kinase receptor closely related to c-Met, has been shown to form a complex with integrin α6β4 that induces hemidesmosome disassembly and the relocation of integrin α6β4 to motility structures.36, 60 Further studies have shown that Ron activation is important in pancreatic carcinoma progression,61, 62 and have confirmed that Ron interacts with the integrin β4 subunit in this setting to disrupt the association between integrin β4 and plectin.60
Cell Survival and Apoptosis
Integrin a6β4 promotes either cell survival or apoptosis, depending on the cellular context. In normal epithelia, integrins are critical to maintaining cellular growth and survival as long as they maintain proper contact with the ECM.63 If anchorage to the ECM is lost, the subsequent loss of integrin signaling can inhibit cell growth and promote a specialized form of apoptosis referred to as anoikis.63 Although the tumor-suppressive functions of the integrin β4 subunit have been identified in bladder,64 colon,65 and breast carcinoma66 cell lines, a number of additional studies have indicated that the β4 integrin promotes cell survival.19, 41, 66, 67, 68 This dichotomy appears to hinge on the expression and mutation status of the p53 tumor suppressor.
Early experiments demonstrated that expression of the integrin β4 subunit in the colon cancer cell line RKO led to increased apoptosis, thus lending support to the notion that integrin α6β4 functions as a tumor suppressor.65 Conversely, expression of the β4 subunit was unable to induce apoptosis in the cell line MDA-MB-435.69 The Mercurio group investigated the mechanism underlying this apparent contradiction and observed that the RKO and MDA-MB-435 cell lines differed in their p53 mutation status. Although RKO cells harbor a wild-type p53, the MDA-MB-435 cell line expresses mutant p53.69 This group demonstrated that integrin α6β4 can trigger apoptosis through p53 activation in cells harboring wild-type p53;69 however, in carcinoma cells deficient in p53, integrin α6β4 promotes cell survival by activating AKT/PKB31, 69 and through translational regulation of VEGF expression.68 These findings suggest that tumors expressing high levels of integrin α6β4 in conjunction with loss of p53 function are resistant to apoptosis and will therefore display a more aggressive clinical course. Interestingly, an association between p53 mutations and integrin α6β4 overexpression is present in a number of aggressive human malignancies, including basal-like breast cancer, head and neck squamous cell carcinoma, and pancreatic ductal adenocarcinoma.50, 70, 71, 72, 73, 74, 75, 76
Angiogenesis
In addition to promoting invasive properties in carcinoma cells, the integrin α6β4 can stimulate invasion and migration of endothelial cells, processes that are necessary for pathologic angiogenesis5 (for review, see Giancotti77 and Wang et al78). Studies using knockout mice carrying a deletion in the signaling domain of the integrin β4 subunit displayed reduced angiogenesis in a retinal neovascularization model, and developed smaller and less vascularized tumors after subcutaneous implantation.5 The same study demonstrated that the integrin β4 subunit could promote both bFGF- and VEGF-induced angiogenesis by enhancing signaling through ERK and NF-κB.
The Role of α6β4 in Transcriptional Regulation
Integrin α6β4 regulates the expression of molecules important for carcinoma invasion and metastasis. The best studied of these include NFAT1, NFAT5, S100A4, ErbB-2, ErbB-3, and autotaxin. The NFATs, or nuclear factors of activated T-cells, are transcriptionally regulated by the α6β4 integrin and drive carcinoma invasion.33 As shown in breast cancer, integrin α6β4-mediated upregulation of NFAT1 leads to increased expression of autotaxin (ENPP2), an enzyme that acts as a motility factor by promoting LPA production.34, 79 Integrin α6β4 can also regulate the expression of ErbB-2 and ErbB-3,30, 54 as mentioned above.
Interestingly, expression of the α6β4 integrin in MDA-MB-435 cells leads to altered expression of over 500 genes.35 One of the most regulated and clinically relevant of these genes is S100A4, a calcium binding protein also known as metastasin-1.80 S100A4 promotes tumor metastases80 and is regulated by integrin α6β4 through NFAT5 activation in conjunction with DNA demethylation of the S100A4 promoter.35 S100A4 interacts with Rhotekin to promote the formation of an S100A4/Rhotekin/RhoA complex, thus allowing RhoA to promote invasion through membrane ruffling.47 Integrin α6β4 has also been shown to negatively regulate the expression of miR-92ab and miR-99ab/100 miRNA families that impact target genes implicated in promoting cell motility.81
INTEGRIN α6β4 EXPRESSION IN HUMAN MALIGNANCIES
Integrin α6β4 was originally identified as a tumor progression antigen by two separate groups, one who termed it tumor-specific antigen-180 (TSP-180)82 and the other who referred to it as the A9 complex.83 Subsequently, the TSP-180 and A9 complexes were shown to be identical to integrin α6β4.84, 85 During the invasive process, integrin α6β4 is released from hemidesmosomes where it can then participate in many of the most aggressive properties of advanced carcinomas. Immunohistochemical staining in patient-derived tissues confirms that in many cancers, expression and localization of integrin α6β4 are altered (as demonstrated in Figure 3).
Altered localization of integrin β4 expression in invasive breast carcinoma. In a dilated duct with benign columnar cell change (left), integrin β4 is expressed in myoepithelial cells surrounding the duct, but is absent in luminal cells. Adjacent nests of invading carcinoma cells (right) display elevated expression of the integrin β4. Immunohistochemical staining was performed using the 439-9B antibody as previously described.72 Brown staining represents positive expression of the integrin β4.
Studies examining integrin β4 expression in patient-derived tissue, in some cancer types, have obtained conflicting results. It is unclear whether these contradictory findings relate to sample size, cancer subtype examined, or the method of detection. Some investigators have reported inconsistent immunohistochemical staining for integrin β4 in formalin-fixed, paraffin-embedded tissue sections.53 Our group found that immunohistochemistry for the integrin β4 subunit is particularly sensitive to the antigen retrieval process.72 In addition, different studies have used a variety of clonal antibodies to detect integrin β4 expression that may partially explain the variability in staining patterns that has been reported. Below, we discuss the clinical associations of integrin α6β4 in various human malignancies, noting where there is disagreement in the literature.
MALIGNANCIES WITH STRONG EVIDENCE THAT INTEGRIN β4 EXPRESSION IS PATHOLOGICALLY SIGNIFICANT
Breast Cancer
In breast cancer, integrin β4 overexpression is associated with aggressive behavior and poor prognosis. Given the challenges described with immunohistochemistry, gene expression profiling provides an excellent alternative that allows for quantitative assessment of integrin β4 expression. One study used gene expression profiling and immunohistochemistry of tissue microarray (TMA) sections to demonstrate that integrin β4 is overexpressed in basal-like breast cancer.50 This finding is particularly notable as basal-like breast cancer is an aggressive subtype that is associated with a notoriously poor prognosis.71 This group further developed a 65-gene signature that included the top genes whose expression was found to correlate with that of integrin β4. This integrin ‘β4 signature’ was shown to be a prognostic indicator that could predict both decreased survival and decreased time to recurrence in four breast cancer cohorts.50 In other studies, integrin β4 mRNA expression was found to positively correlate with nuclear grade and tumor size,53 and elevated integrin α6β4 protein expression has been found to associate with decreased survival.86 Coexpression of integrin α6β4 and Net1, a RhoA guanine nucleotide exchange factor, has also been associated with decreased distant metastasis-free survival.52
In contrast to the findings described above, a number of early reports indicated that integrin β4 expression is absent or rare in breast cancers. This observation may be because of difficulties with immunohistochemistry on frozen specimens, or may be related to the fact that these studies were performed before the modern subclassification of breast cancers was developed. As integrin β4 overexpression is more frequent in triple-negative breast cancers and triple-negative tumors represent a minority of breast cancers, these studies may not have included an adequate number of triple-negative cases to detect integrin β4 overexpression. Two of these early investigations report that integrin β4 expression was absent in all invasive breast carcinomas examined,82, 87 whereas another found strong integrin β4 staining in only a small subset of breast tumors.88 Others have reported that integrin β4 is redistributed over the cell surface in select breast carcinomas.89 Integrin β4 expression in ductal carcinoma in situ (DCIS) is also reportedly rare, with expression identified in only 20% of cases in one study.90 According to another report, integrin β4 expression was absent in the neoplastic cells of DCIS and detected only in residual myoepithelium.87 Given more recent evidence using gene expression profiling, it is reasonable to conclude that at least a certain subset of breast tumors overexpress integrin β4, including basal-like breast cancers.
Bladder Cancer
Studies investigating integrin β4 expression in bladder cancer demonstrate that it is overexpressed in a proportion of transitional cell carcinomas, and suggest its use as a prognostic marker. An early study reported that in normal urothelium integrin α6β4 is expressed in the basal layer of urothelial cells where this expression is highly polarized and localized to the lamina propria junction. The authors then examined integrin α6β4 expression in 10 low-stage bladder cancers, where they found increased, nonpolarized expression in 80% of tumors.91 A subsequent study by the same group examined integrin α6β4 expression in bladder tumors from 57 patients; each case was categorized as having negative, weak, or strong expression of integrin α6β4, where weak was defined as expression that most closely resembled that of normal urothelium.92 They found that patients with weak integrin α6β4 expression had improved survival compared with patients with either strong or negative expression.92 Another study examined integrin β4 expression in a cohort of patients with nonmuscle invasive bladder cancer and found that integrin β4 expression levels were an independent predictor of intravesical recurrence after transurethral resection.93
Cervical Cancer
Integrin β4 expression in cervical lesions has been examined primarily in cervical intraepithelial neoplasia (CIN) and squamous cell carcinoma. Multiple reports have confirmed that the integrin β4 is strongly expressed in invasive squamous cell carcinomas of the cervix.94, 95, 96 Interestingly, integrin β4 expression positively correlates with the degree of squamous atypia in CIN.94, 95 In a study of cervical biopsies from 35 patients, integrin β4 expression was present only in cells of the basal and parabasal layers of normal ectocervical mucosa, and this pattern was maintained in flat condylomas, hyperplastic epithelium, and in CIN I lesions.94 Interestingly, in CIN II–III, integrin β4 expression was present throughout the entire thickness of the epithelium, with strong staining observed toward the superficial surface. Furthermore, expression of integrin β4 was identified in 90% of cervical carcinomas studied, where expression was diffusely present in the invasive nests.94
A larger study investigated integrin β4 expression in 40 cervical biopsies. The authors describe that in normal ectocervical mucosa, integrin β4 expression was localized to the basal aspect of cells in the basal layer of epithelium.95 They found that in CIN, expression of the integrin β4 followed the distribution of atypia: for example, in CIN II, β4 was expressed throughout the lower 2/3 of the epithelial thickness, whereas in CIN III, β4 was expressed throughout the full epithelial thickness. These findings suggest that the integrin β4 may play an early role in promoting the survival and growth of preinvasive neoplasms.
A third study examined expression of the integrin β4 in 20 cases of invasive cervical carcinoma and 23 cases of CIN III.96 The invasive cervical carcinomas included examples of well, moderately, and poorly differentiated squamous cell carcinoma, as well as three endocervical adenocarcinomas. Diffuse expression of the integrin β4 was identified in all 20 cases of invasive carcinoma. In addition, integrin β4 expression was identified in all epithelial layers in 65% of CIN III lesions.96
Head and Neck Cancer
In squamous cell carcinomas of the head and neck, integrin β4 is commonly overexpressed. An early study of 82 patients with SCC of the head and neck found that strong expression of the UM-A9 antigen (later identified as the α6β4 integrin) was associated with early relapse and decreased patient survival.97 In another study, integrin β4 expression was found to be upregulated in SCCs when compared with normal squamous mucosa, although an association was found between loss of integrin β4 expression and the presence of nodal metastases.98
Multiple studies using gene expression profiling have confirmed that integrin β4 gene expression levels are prognostically significant in SCCs of the head and neck. One of these reports demonstrated that integrin β4 gene expression was associated with decreased survival in a cohort of 66 patients.99 In a larger study, increased integrin β4 gene expression was associated with the presence of lymph node metastases, distant metastases, and patient death on univariate analysis, and was an independent predictor of distant metastases on multivariate analysis.100
Lung Cancer
Integrin β4 is overexpressed in non-small-cell lung cancers, and expression is particularly high in pulmonary squamous cell carcinomas. An early study investigated expression of the integrin α6β4 in a series of patient-derived lung cancers and found moderate to strong expression in all of the squamous cell carcinomas (N=36) and adenocarcinomas (N=23) tested, although expression was notably absent in all (N =10) of the small cell carcinomas examined.101 Integrin β4 expression was identified in a number of non-small-cell carcinoma cell lines (A431, A549, DG3), but was absent in the single small cell carcinoma cell line tested (AE2).101
In a different study that included uninvolved normal lung tissue, normal alveolar epithelial cells were found to be negative for integrin β4 expression, and instead exhibited expression of the α1β1 and α3β1 laminin receptors. They further found that bronchial and bronchiolar epithelium exhibited weak and inconsistent integrin β4 expression that was localized to the basement membrane interface. In squamous cell carcinomas of the lung, integrin β4 expression was intense and localized to the tumor–stroma interface.102 In this same study, integrin β4 expression was identified in large cell carcinomas of the lung, but was found to be absent in neuroendocrine carcinomas. Patriarca et al103 also found that in normal bronchial epithelium, integrin β4 expression was localized to the basal surface of cells in a linear pattern. They found strong and extensive staining for the integrin β4 in 85% of squamous cell carcinomas (N=20), but found positive staining in only 25% pulmonary adenocarcinomas studied (N=20). In a complementary study using molecular profiling, integrin β4 was upregulated in lung squamous cell carcinomas when compared with adenocarcinomas, and this was confirmed using both immunohistochemistry and in situ hybridization.104
Pancreatic Cancer
Integrin β4 is overexpressed in pancreatic carcinomas, and is also a marker of poor prognosis. Using gene expression profiling, Logsdon et al105 first determined that integrin β4 is upregulated in pancreatic adenocarcinoma when compared with normal pancreatic tissue and chronic pancreatitis tissue samples, a finding that was confirmed by others.106 In order to validate these findings, the Logsdon group performed immunohistochemistry for the integrin β4 subunit and a number of other candidate genes in 28 pancreatic adenocarcinomas, where they found that all cases had strong integrin β4 expression.105 In another report, Gleason et al107 found moderate to strong integrin β4 staining in 92% (N=48) of pancreatic adenocarcinomas that were evaluated using immunohistochemistry; they also found that in normal pancreas, integrin β4 staining was weak and expressed only along the basement membranes of large ducts.
Integrin β4 expression has also been studied in pancreatic intraepithelial neoplasia, a noninvasive precursor lesion to pancreatic adenocarcinoma.108 In a comprehensive study of pancreatic lesions, Cruz-Monserrate et al72 determined that integrin β4 overexpression is present in the early stages of pancreatic adenocarcinoma development. As reported previously, they found that in normal pancreas, integrin β4 expression is localized to the interface between ductal epithelial cells and the basement membrane.72 Upregulation of integrin β4 expression was observed in 92% (N=113) of pancreatic adenocarcinomas studied, and distinguished pancreatic cancer from pancreatitis. Furthermore, overexpression and altered localization of the integrin β4 was identified in all pancreatic intraepithelial neoplasia lesions ranging from grade 1A to grade 3.72
Recently, elevated integrin β4 expression was shown to associate with reduced overall survival among pancreatic adenocarcinoma patients (N=134), where it was found to have independent prognostic significance on multivariate analysis. Interestingly, elevated integrin β4 expression was also found to correlate with a number of EMT hallmarks, including solitary cell infiltration, reduced expression of E-cadherin, and increased expression of vimentin.109 Pancreatic adenocarcinoma has one of the poorest prognoses of all epithelial malignancies; the fact that integrin β4 is highly expressed in these tumors provides further evidence for its role in aggressive neoplasms.
Thyroid Cancer
Thyroid carcinomas are unique in that they are one of the few malignancies to exhibit neoexpression of integrin α6β4 during cancer progression. Although expression of integrin α6β4 is absent in normal and adenomatous follicular cells, strong expression has been observed in both follicular and papillary thyroid carcinomas.110 Similar findings have been reported using flow cytofluorometry, where expression of integrins such as α1β1 and α6β1 was found in normal thyroid and tumor specimens, and integrin α6β4 expression was found only in thyroid carcinomas and carcinoma cell lines.111 Others have confirmed neoexpression of integrin α6β4 in thyroid carcinoma tissue112, 113 and have also found that it is expressed in anaplastic thyroid carcinoma, an aggressive and poorly differentiated malignancy.112
MALIGNANCIES IN WHICH INTEGRIN β4 EXPRESSION HAS CONTROVERSIAL OR UNDETERMINED SIGNIFICANCE
Colon Cancer
One of the earliest studies to investigate α6β4 expression in human malignancies found that the α6β4 integrin was expressed in colon cancer.82 However, additional reports have been controversial. One study investigating integrin β4 expression in colorectal carcinomas reported that integrin β4 expression was reduced during malignant transformation.114 This group found that although expression of the integrin β4 subunit was maintained at the basal epithelial cell membrane in normal colonic mucosa and in colonic adenomas, expression of the integrin β4 subunit was reduced or absent in most colorectal carcinomas, but was maintained in well-differentiated colon cancer.114 Similar findings were reported in another study, where expression of the integrin β4 subunit was reduced or absent in most colon carcinomas examined.115
A contrasting study demonstrated that integrin β4 is overexpressed in a majority of colon carcinomas and that its expression is elevated in high-stage, poorly differentiated cancers.116 These findings are supported by subsequent work examining integrin β4 expression in colorectal carcinomas using double immunofluorescence and RT-QPCR, where integrin β4 protein and transcript levels were increased in colorectal carcinoma when compared with normal tissue.117 Data from our laboratory also confirm the observation that integrin β4 levels are particularly high in colon cancer cell lines and patient-derived tissues (KL O'Connor, unpublished observation). Additional studies are needed to confirm conclusively that integrin β4 is overexpressed in patient-derived colorectal carcinoma tissues. Gene expression profiling or analysis of existing data sets will help to clarify this issue.
Ovarian Cancer
In benign ovary, integrin β4 is basally located in surface and cyst lining epithelium.75 One of the few studies investigating integrin β4 expression in ovarian cancer found strong basal expression in all of the epithelial ovarian tumors studied, and also found that integrin β4 was expressed in malignant cells within the ascitic fluid in three out of nine cases.75 A second report found basally polarized integrin β4 expression in normal ovary and in 40% of serous ovarian carcinomas examined. The authors further noted that expression of both integrin α6 and β4 subunits were positively correlated with laminin expression.74 Interestingly, serous ovarian cancer has a similar genomic profile to basal-like breast cancer, with both subtypes displaying frequent loss of TP53, BRCA1, and RB1, suggesting that integrin β4 may play an important role in both types of cancer.70 Further work will be needed to fully characterize integrin β4 expression in ovarian neoplasia and to determine how expression associates with prognosis.
Prostate Cancer
Early reports indicated that integrin β4 is downregulated in prostatic adenocarcinoma, and in one investigation, expression of integrin β4 was absent in all prostate cancers examined (N=20).118 Multiple additional studies have reported that integrin β4 expression is lost during the transition from benign epithelium to prostatic adenocarcinoma.119, 120, 121, 122 A potential explanation for this phenomenon is that androgen receptor expression has been reported to cause downregulation of integrin α6β4.123 The assertion that integrin β4 is downregulated in prostate cancer was challenged by a report demonstrating that integrin β4 mRNA is overexpressed in a subset of prostate carcinomas using gene expression data sets and a DNA microarray.124 The authors of this study also investigated integrin β4 protein expression using immunohistochemistry and found overexpression in 35% of invasive cancers and in a number of metastatic lesions.124 More recently, a population of integrin β4-positive circulating tumor cells was identified in the peripheral blood of patients with castration-resistant prostate cancer.125 Overall, prostate cancer is one cancer in which integrin α6β4 is suggested to be downregulated. However, given recent findings, it will be important to determine how residual or enhanced integrin α6β4 expression, in the minority of cases that overexpress it, associates with clinical parameters.
Tumors of the Central Nervous System
Expression of integrin α6β4 has not been extensively studied in glial tumors; however, there is evidence demonstrating that integrin α6β4 is expressed in astrocytomas, oligodendrogliomas, glioblastomas, and a number of glioma cell lines. Integrin β4 expression has been identified in reactive astrocytes126 as well as in subependymal glia, choroid plexus, and meningothelial cells.127 One study found that integrin β4 expression is higher in astrocytomas and glioblastomas when compared with benign astrocytes.126 A larger study investigated expression of the integrin β4 subunit in a series of astrocytomas and oligodendrogliomas where they found that integrin β4 expression was slightly higher in oligodendrogliomas.128 Further studies will be needed to determine how integrin α6β4 relates to glioma stage and prognosis.
Sarcomas
Integrin β4 overexpression has been described in high-grade osteosarcomas and there is evidence it may play a role in promoting a metastatic phenotype by interacting with ezrin.129 Although integrin β4 is expressed in benign endothelial cells, its expression appears to be reduced in angiosarcomas and other vascular tumors.130 Integrin β4 staining is also reportedly absent in rhabdomyosarcomas, ganglioneuroblastomas, primitive peripheral neuroectodermal tumors, and Ewing’s sarcomas.131
CONCLUSIONS
Integrin β4 is commonly overexpressed in high-grade malignancies. Notably, there is strong evidence that integrin β4 is overexpressed in tumors of the bladder, cervix, lung, pancreas, and thyroid, and in basal-like breast cancer. In addition, integrin β4 overexpression has been identified as an adverse prognostic marker in tumors of the breast, pancreas, and in squamous cell carcinomas of the head and neck. The reason for these poor prognoses stems from the ability of the integrin α6β4 to promote several key hallmarks of cancer, including the capacity to sustain proliferative signaling, evade apoptosis, promote tissue invasion and metastasis, and stimulate angiogenesis. Notably, mutation or inactivation of p53 is one mechanism that allows integrin α6β4 to promote cell survival and amplify signaling through a number of invasive and proliferative pathways. Integrin α6β4 can trigger apoptosis in cells harboring wild-type p53; however, in carcinoma cells deficient in p53, integrin α6β4 promotes cell survival.31, 68, 69 Interestingly, tumors with a high frequency of p53 mutations (pancreatic adenocarcinoma, basal-like breast cancer, squamous cell carcinomas of the head and neck) tend to also display integrin β4 overexpression. In these tumor types, there is evidence that integrin β4 expression is clinically significant and correlates with a poor prognosis. This observation may partially explain why integrin β4 expression is prognostically significant in some, but not all tumor types.
In a number of malignancies, it is still unclear whether expression of integrin β4 is elevated or reduced. In tumors of the breast, prostate, and colon, a number of studies examining integrin β4 expression have obtained conflicting results, with some authors reporting that integrin β4 is overexpressed and others reporting a reduction in integrin β4 expression. These disparate findings may relate to differences in sample size, antibody usage, tissue processing, or antigen retrieval process. In breast cancer, use of gene expression databanks and modern sub-classifications has helped to clarify the association of integrin β4 with basal-like breast cancer. The integrin β4 is highly expressed in the basal cell layer in many benign epithelial tissues. During carcinoma progression, localization of the integrin α6β4 is altered, as has been described in tumors of the breast, bladder, cervix, and pancreas.72, 89, 91, 94, 95 It is likely that altered localization of the α6β4 integrin and its concurrent release from hemidesmosomes are as important in carcinoma progression as overexpression, thus altered integrin α6β4 localization should be studied carefully in these malignancies. Additional investigations using tissue microarrays or larger patient cohorts will be needed to determine whether integrin β4 associates with progression in these cancers. Gene expression profiling will provide another mechanism that allows for more in-depth investigation of integrin β4 expression and its prognostic significance in human malignancies, and will allow for validation of immunohistochemical findings.
References
Hynes RO . Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–687.
Guo W, Giancotti FG . Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5:816–826.
Barczyk M, Carracedo S, Gullberg D . Integrins. Cell Tissue Res 2010;339:269–280.
Lipscomb EA, Mercurio AM . Mobilization and activation of a signaling competent alpha6beta4integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev 2005;24:413–423.
Nikolopoulos SN, Blaikie P, Yoshioka T et al. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 2004;6:471–483.
Shaw LM, Rabinovitz I, Wang HH et al. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997;91:949–960.
Rabinovitz I, Toker A, Mercurio AM . Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 1999;146:1147–1160.
Mainiero F, Pepe A, Yeon M et al. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J Cell Biol 1996;134:241–253.
O'Connor KL, Shaw LM, Mercurio AM . Release of cAMP gating by the alpha6beta4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol 1998;143:1749–1760.
Hogervorst F, Kuikman I, von dem Borne AE et al. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J 1990;9:765–770.
Suzuki S, Naitoh Y . Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J 1990;9:757–763.
de Pereda JM, Wiche G, Liddington RC . Crystal structure of a tandem pair of fibronectin type III domains from the cytoplasmic tail of integrin alpha6beta4. EMBO J 1999;18:4087–4095.
Litjens SH, de Pereda JM, Sonnenberg A . Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 2006;16:376–383.
Margadant C, Frijns E, Wilhelmsen K et al. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 2008;20:589–596.
de Pereda JM, Ortega E, Alonso-Garcia N et al. Advances and perspectives of the architecture of hemidesmosomes: lessons from structural biology. Cell Adh Migr 2009;3:361–364.
Borradori L, Sonnenberg A . Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647–656.
Rezniczek GA, de Pereda JM, Reipert S et al. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J Cell Biol 1998;141:209–225.
Vidal F, Aberdam D, Miquel C et al. Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet 1995;10:229–234.
Dowling J, Yu QC, Fuchs E . Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 1996;134:559–572.
Tsuruta D, Hashimoto T, Hamill KJ et al. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J Dermatol Sci 2011;62:1–7.
Mariotti A, Kedeshian PA, Dans M et al. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–458.
Rabinovitz I, Mercurio AM . The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol 1997;139:1873–1884.
O'Connor KL, Chen M, Towers LN . Integrin alpha6beta4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am J Physiol Cell Physiol 2012;302:C605–C614.
O'Connor KL, Nguyen BK, Mercurio AM . RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol 2000;148:253–258.
Werner ME, Chen F, Moyano JV et al. Caspase proteolysis of the integrin beta4 subunit disrupts hemidesmosome assembly, promotes apoptosis, and inhibits cell migration. J Biol Chem 2007;282:5560–5569.
Beausejour M, Thibodeau S, Demers MJ et al. Suppression of anoikis in human intestinal epithelial cells: differentiation state-selective roles of alpha2beta1, alpha3beta1, alpha5beta1, and alpha6beta4 integrins. BMC Cell Biol 2013;14:53.
Guo W, Pylayeva Y, Pepe A et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006;126:489–502.
Trusolino L, Bertotti A, Comoglio PM . A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 2001;107:643–654.
Chung J, Yoon SO, Lipscomb EA et al. The Met receptor and alpha 6 beta 4 integrin can function independently to promote carcinoma invasion. J Biol Chem 2004;279:32287–32293.
Folgiero V, Bachelder RE, Bon G et al. The alpha6beta4 integrin can regulate ErbB-3 expression: implications for alpha6beta4 signaling and function. Cancer Res 2007;67:1645–1652.
Bachelder RE, Ribick MJ, Marchetti A et al. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol 1999;147:1063–1072.
Mainiero F, Murgia C, Wary KK et al. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J 1997;16:2365–2375.
Jauliac S, Lopez-Rodriguez C, Shaw LM et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 2002;4:540–544.
Chen M, O'Connor KL . Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 2005;24:5125–5130.
Chen M, Sinha M, Luxon BA et al. Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem 2009;284:1484–1494.
Santoro MM, Gaudino G, Marchisio PC . The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell 2003;5:257–271.
Chao C, Lotz MM, Clarke AC et al. A function for the integrin alpha6beta4 in the invasive properties of colorectal carcinoma cells. Cancer Res 1996;56:4811–4819.
Yuan TL, Cantley LC . PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008;27:5497–5510.
Gambaletta D, Marchetti A, Benedetti L et al. Cooperative signaling between alpha(6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem 2000;275:10604–10610.
Gagnoux-Palacios L, Dans M, van't Hof W et al. Compartmentalization of integrin alpha6beta4 signaling in lipid rafts. J Cell Biol 2003;162:1189–1196.
Bhatia V, Mula RV, Weigel NL et al. Parathyroid hormone-related protein regulates cell survival pathways via integrin alpha6beta4-mediated activation of phosphatidylinositol 3-kinase/Akt signaling. Mol Cancer Res 2009;7:1119–1131.
Shaw LM . Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol 2001;21:5082–5093.
Machacek M, Hodgson L, Welch C et al. Coordination of Rho GTPase activities during cell protrusion. Nature 2009;461:99–103.
Zahir N, Lakins JN, Russell A et al. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J Cell Biol 2003;163:1397–1407.
Cruz-Monserrate Z, O'Connor KL . Integrin α6β4 promotes migration, invasion through Tiam1 upregulation and subsequent Rac activation. Neoplasia 2008;10:408–417.
O'Connor KL, Chen M . Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 2013;4:1–7.
Chen M, Bresnick AR, O'Connor KL . Coupling S100A4 to Rhotekin alters Rho signaling output in breast cancer cells. Oncogene 2013;32:3754–3764.
Soung YH, Chung J . Curcumin inhibition of the functional interaction between integrin alpha6beta4 and the epidermal growth factor receptor. Mol Cancer Ther 2011;10:883–891.
Falcioni R, Antonini A, Nistico P et al. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res 1997;236:76–85.
Lu S, Simin K, Khan A et al. Analysis of integrin beta4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res 2008;14:1050–1058.
Folgiero V, Avetrani P, Bon G et al. Induction of ErbB-3 expression by alpha6beta4 integrin contributes to tamoxifen resistance in ERbeta1-negative breast carcinomas. PLoS One 2008;3:e1592.
Gilcrease MZ, Kilpatrick SK, Woodward WA et al. Coexpression of alpha6beta4 integrin and guanine nucleotide exchange factor Net1 identifies node-positive breast cancer patients at high risk for distant metastasis. Cancer Epidemiol Biomarkers Prev 2009;18:80–86.
Diaz LK, Cristofanilli M, Zhou X et al. Beta4 integrin subunit gene expression correlates with tumor size and nuclear grade in early breast cancer. Mod Pathol 2005;18:1165–1175.
Yoon SO, Shin S, Lipscomb EA . A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: the alpha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res 2006;66:2732–2739.
Akiyama T, Sudo C, Ogawara H et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644–1646.
Roy V, Perez EA . Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER-2-positive breast cancer. Oncologist 2009;14:1061–1069.
Olayioye MA, Neve RM, Lane HA et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159–3167.
Wallasch C, Weiss FU, Niederfellner G et al. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J 1995;14:4267–4275.
Hellyer NJ, Cheng K, Koland JG . ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J 1998;333 (Pt 3):757–763.
Yu PT, Babicky M, Jaquish D et al. The RON-receptor regulates pancreatic cancer cell migration through phosphorylation-dependent breakdown of the hemidesmosome. Int J Cancer 2012;131:1744–1754.
Thomas RM, Toney K, Fenoglio-Preiser C et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res 2007;67:6075–6082.
Logan-Collins J, Thomas RM, Yu P et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res 2010;70:1130–1140.
Ruoslahti E, Reed JC . Anchorage dependence, integrins, and apoptosis. Cell 1994;77:477–478.
Kim SY, Bachman NJ, Nair TS et al. Beta 4 integrin transfection of UM-UC-2 (human bladder carcinoma) cells: stable expression of a spontaneous cytoplasmic truncation mutant with rapid loss of clones expressing intact beta 4. Cancer Res 1997;57:38–42.
Clarke AS, Lotz MM, Chao C et al. Activation of the p21 pathway of growth arrest and apoptosis by the beta 4 integrin cytoplasmic domain. J Biol Chem 1995;270:22673–22676.
Sun H, Santoro SA, Zutter MM . Downstream events in mammary gland morphogenesis mediated by reexpression of the alpha2beta1 integrin: the role of the alpha6 and beta4 integrin subunits. Cancer Res 1998;58:2224–2233.
Lv X, Su L, Yin D et al. Knockdown of integrin beta4 in primary cultured mouse neurons blocks survival and induces apoptosis by elevating NADPH oxidase activity and reactive oxygen species level. Int J Biochem Cell Biol 2008;40:689–699.
Lipscomb EA, Simpson KJ, Lyle SR et al. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res 2005;65:10970–10976.
Bachelder RE, Marchetti A, Falcioni R et al. Activation of p53 function in carcinoma cells by the alpha6beta4 integrin. J Biol Chem 1999;274:20733–20737.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70.
Rakha EA, Reis-Filho JS, Ellis IO . Basal-like breast cancer: a critical review. J Clin Oncol 2008;26:2568–2581.
Cruz-Monserrate Z, Qiu S, Evers BM et al. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Mod Pathol 2007;20:656–667.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–615.
Skubitz AP, Bast RC Jr, Wayner EA et al. Expression of alpha 6 and beta 4 integrins in serous ovarian carcinoma correlates with expression of the basement membrane protein laminin. Am J Pathol 1996;148:1445–1461.
Bridges JE, Englefield P, Boyd IE et al. Expression of integrin adhesion molecules in normal ovary and epithelial ovarian tumors. Int J Gynecol Cancer 1995;5:187–192.
Pellegata NS, Sessa F, Renault B et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res 1994;54:1556–1560.
Giancotti FG . Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci 2007;28:506–511.
Wang L, Dong Z, Zhang Y et al. The roles of integrin beta4 in vascular endothelial cells. J Cell Physiol 2012;227:474–478.
Houben AJ, Moolenaar WH . Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 2011;30:557–565.
Boye K, Maelandsmo GM . S100A4 and metastasis: a small actor playing many roles. Am J Pathol 2010;176:528–535.
Gerson KD, Maddula VS, Seligmann BE et al. Effects of beta4 integrin expression on microRNA patterns in breast cancer. Biol Open 2012;1:658–666.
Falcioni R, Sacchi A, Resau J et al. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res 1988;48:816–821.
Kimmel KA, Carey TE . Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res 1986;46:3614–3623.
Kennel SJ, Foote LJ, Falcioni R et al. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem 1989;264:15515–15521.
Van Waes C, Kozarsky KF, Warren AB et al. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res 1991;51:2395–2402.
Tagliabue E, Ghirelli C, Squicciarini P et al. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res 1998;4:407–410.
Koukoulis GK, Virtanen I, Korhonen M et al. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol 1991;139:787–799.
Pignatelli M, Cardillo MR, Hanby A et al. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol 1992;23:1159–1166.
Natali PG, Nicotra MR, Botti C et al. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer 1992;66:318–322.
Hanby AM, Gillett CE, Pignatelli M et al. Beta 1 and beta 4 integrin expression in methacarn and formalin-fixed material from in situ ductal carcinoma of the breast. J Pathol 1993;171:257–262.
Grossman HB, Washington RW Jr., Carey TE et al. Alterations in antigen expression in superficial bladder cancer. J Cell Biochem Suppl 1992;16I:63–68.
Grossman HB, Lee C, Bromberg J et al. Expression of the alpha6beta4 integrin provides prognostic information in bladder cancer. Oncol Rep 2000;7:13–16.
Behnsawy HM, Miyake H, Abdalla MA et al. Expression of integrin proteins in non-muscle-invasive bladder cancer: significance of intravesical recurrence after transurethral resection. BJU Int 2011;107:240–246.
Carico E, French D, Bucci B et al. Integrin beta 4 expression in the neoplastic progression of cervical epithelium. Gynecol Oncol 1993;49:61–66.
Aplin JD, Dawson S, Seif MW . Abnormal expression of integrin alpha 6 beta 4 in cervical intraepithelial neoplasia. Br J Cancer 1996;74:240–245.
Jeffers MD, Paxton J, Bolger B et al. E-cadherin and integrin cell adhesion molecule expression in invasive and in situ carcinoma of the cervix. Gynecol Oncol 1997;64:481–486.
Wolf GT, Carey TE, Schmaltz SP et al. Altered antigen expression predicts outcome in squamous cell carcinoma of the head and neck. J Natl Cancer Inst 1990;82:1566–1572.
Eriksen JG, Steiniche T, Sogaard H et al. Expression of integrins and E-cadherin in squamous cell carcinomas of the head and neck. APMIS 2004;112:560–568.
Kurokawa A, Nagata M, Kitamura N et al. Diagnostic value of integrin alpha3, beta4, and beta5 gene expression levels for the clinical outcome of tongue squamous cell carcinoma. Cancer 2008;112:1272–1281.
Nagata M, Noman AA, Suzuki K et al. ITGA3 and ITGB4 expression biomarkers estimate the risks of locoregional and hematogenous dissemination of oral squamous cell carcinoma. BMC Cancer 2013;13:410.
Mariani Costantini R, Falcioni R, Battista P et al. Integrin (alpha 6/beta 4) expression in human lung cancer as monitored by specific monoclonal antibodies. Cancer Res 1990;50:6107–6112.
Koukoulis GK, Warren WH, Virtanen I et al. Immunolocalization of integrins in the normal lung and in pulmonary carcinomas. Hum Pathol 1997;28:1018–1025.
Patriarca C, Alfano RM, Sonnenberg A et al. Integrin laminin receptor profile of pulmonary squamous cell and adenocarcinomas. Hum Pathol 1998;29:1208–1215.
Boelens MC, van den Berg A, Vogelzang I et al. Differential expression and distribution of epithelial adhesion molecules in non-small cell lung cancer and normal bronchus. J Clin Pathol 2007;60:608–614.
Logsdon CD, Simeone DM, Binkley C et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res 2003;63:2649–2657.
Crnogorac-Jurcevic T, Missiaglia E, Blaveri E et al. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol 2003;201:63–74.
Gleason B, Adley B, Rao MS et al. Immunohistochemical detection of the beta4 integrin subunit in pancreatic adenocarcinoma. J Histochem Cytochem 2005;53:799–801.
Hruban RH, Maitra A, Goggins M . Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008;1:306–316.
Masugi Y, Yamazaki K, Emoto K et al. Upregulation of integrin beta4 promotes epithelial-mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Lab Invest 2015;95:308–319.
Serini G, Trusolino L, Saggiorato E et al. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J Natl Cancer Inst 1996;88:442–449.
Montuori N, Muller F, De Riu S et al. Laminin receptors in differentiated thyroid tumors: restricted expression of the 67-kilodalton laminin receptor in follicular carcinoma cells. J Clin Endocrinol Metab 1999;84:2086–2092.
Dahlman T, Grimelius L, Wallin G et al. Integrins in thyroid tissue: upregulation of alpha2beta1 in anaplastic thyroid carcinoma. Eur J Endocrinol 1998;138:104–112.
Kitajiri S, Hosaka N, Hiraumi H et al. Increased expression of integrin beta-4 in papillary thyroid carcinoma with gross lymph node metastasis. Pathol Int 2002;52:438–441.
Stallmach A, von Lampe B, Matthes H et al. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut 1992;33:342–346.
Sordat I, Bosman FT, Dorta G et al. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 1998;185:44–52.
Falcioni R, Turchi V, Vitullo P et al. Integrin beta-4 expression in colorectal-cancer. Int J Oncol 1994;5:573–578.
Ni H, Dydensborg AB, Herring FE et al. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005;24:6820–6829.
Knox JD, Cress AE, Clark V et al. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol 1994;145:167–174.
Cress AE, Rabinovitz I, Zhu W et al. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228.
Davis TL, Cress AE, Dalkin BL et al. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate 2001;46:240–248.
Nagle RB, Hao J, Knox JD et al. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol 1995;146:1498–1507.
Allen MV, Smith GJ, Juliano R et al. Downregulation of the beta4 integrin subunit in prostatic carcinoma and prostatic intraepithelial neoplasia. Hum Pathol 1998;29:311–318.
Bonaccorsi L, Carloni V, Muratori M et al. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology 2000;141:3172–3182.
Yoshioka T, Otero J, Chen Y et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. J Clin Invest 2013;123:682–699.
Chakraborty G, Gadiya M, Kossai M et al Inactivation of Neogenin Promotes Castration Resistance and Bone Metastasis in Prostate Cancer Models. American Association for Cancer Research: Philadelphia, PA, 2015.
Previtali S, Quattrini A, Nemni R et al. Alpha6 beta4 and alpha6 beta1 integrins in astrocytomas and other CNS tumors. J Neuropathol Exp Neurol 1996;55:456–465.
Paulus W, Baur I, Schuppan D et al. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol 1993;143:154–163.
Belot N, Rorive S, Doyen I et al. Molecular characterization of cell substratum attachments in human glial tumors relates to prognostic features. Glia 2001;36:375–390.
Wan X, Kim SY, Guenther LM et al. Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene 2009;28:3401–3411.
Mechtersheimer G, Barth T, Hartschuh W et al. In situ expression of beta 1, beta 3 and beta 4 integrin subunits in non-neoplastic endothelium and vascular tumours. Virchows Arch 1994;425:375–384.
Barth T, Moller P, Mechtersheimer G . Differential expression of beta 1, beta 3 and beta 4 integrins in sarcomas of the small, round, blue cell category. Virchows Arch 1995;426:19–25.
Acknowledgements
This work was supported by National Institutes of Health grants T32 CA160003 (to RLS) and R01 CA109136 (to KLO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This review provides a comprehensive summary of integrin α6β4 expression patterns and their prognostic significance in human malignancies, and describes key signaling functions of the integrin α6β4 that contribute to its ability to promote invasion, migration, and survival in carcinoma cells.
Rights and permissions
About this article
Cite this article
Stewart, R., O'Connor, K. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest 95, 976–986 (2015). https://doi.org/10.1038/labinvest.2015.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.82
This article is cited by
-
Induction of promyelocytic leukemia zinc finger protein by miR-200c-3p restores sensitivity to anti-androgen therapy in androgen-refractory prostate cancer and inhibits the cancer progression via down-regulation of integrin α3β4
Cellular Oncology (2023)
-
Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance
Oncogene (2021)
-
EGFR-dependent tyrosine phosphorylation of integrin β4 is not required for downstream signaling events in cancer cell lines
Scientific Reports (2021)
-
Deletion of TMEM268 inhibits growth of gastric cancer cells by downregulating the ITGB4 signaling pathway
Cell Death & Differentiation (2019)
-
Integrin α6β4-Src-AKT signaling induces cellular senescence by counteracting apoptosis in irradiated tumor cells and tissues
Cell Death & Differentiation (2019)