Abstract
Bronchial asthma and chronic obstructive pulmonary disease (COPD) are chronic diseases that are associated with inflammation and structural changes in the airways and lungs. Recent findings have implicated Wnt pathways in critically regulating inflammatory responses, especially in asthma. Furthermore, canonical and noncanonical Wnt pathways are involved in structural changes such as airway remodeling, goblet cell metaplasia, and airway smooth muscle (ASM) proliferation. In COPD, Wnt pathways are not only associated with structural changes in the airways but also involved in the development of emphysema. The present review summarizes the role and function of the canonical and noncanonical Wnt pathway with regard to airway inflammation and structural changes in asthma and COPD. Further identification of the role and function of different Wnt molecules and pathways could help to develop novel therapeutic options for these diseases.
Similar content being viewed by others
Main
Obstructive airway diseases are the most prevalent chronic lung diseases. Two entities, bronchial asthma and chronic obstructive pulmonary disease (COPD), are described under this term. Asthma is defined as the presence of airway obstruction, increased reactivity to inhaled bronchoconstrictors (airway hyperreactivity), and airway inflammation (http://www.ginaasthma.org). This syndrome can be found in many different clinical presentations, and various clinically or immunologically categorized phenotypes have been described in the past years.1 COPD is characterized by persistent airflow limitation, which is usually progressive over time (http://www.goldcopd.com). In addition, inflammatory and structural changes can be found in the airways and lung. This can be apparent as small airway disease (obstructive bronchiolitis) or destruction of lung parenchyma (emphysema). Chronic airway inflammation has been described in asthma as well as in COPD. However, the underlying cellular mechanisms are distinctively differing between asthma and COPD.
Several different asthma phenotypes have been described. It has been speculated that these asthma phenotypes also differ in their pathobiology.2 By far the best understood immunological pathways are in allergic asthma. Indeed, in allergen-induced airway inflammation, T-helper type 2 (Th2) cells have a pivotal role. This type of asthma often develops in childhood and is associated with atopic constitution and with other manifestations of atopic diseases such as allergic rhinitis, atopic dermatitis, and food allergy. Th2 cell polarization and also activation is dependent on dendritic cells (DCs), which present the specific allergens. Indeed, following allergen exposure in the lung, the protein is taken up by DCs, which migrate to the draining lymph node, where antigen-specific T cells are activated. These cells then migrate to the lung and induce an inflammatory reaction by the secretion of proinflammatory mediators such as the Th2 cytokines (interleukin-4 (IL-4), IL-5, and IL-13). This inflammatory response is characterized by the presence of eosinophils. A certain transcription factor, the trans-acting T-cell-specific transcription factor GATA-3, has been found to be central for the function of Th2 cells,3 and recent findings have strengthened the role of GATA-3 as a master switch for allergen-induced asthma. Indeed, direct blockade of GATA-3 resulted in decreased early and late responses in patients with allergic asthma following allergen challenge.4 In recent years, there has also been increasing attention to the role of the airway epithelium in mediating the inflammatory responses in the airways. It is now well recognized that epithelial-derived mediators such as TLSP and IL-33 are critically involved in the development and chronicity of allergic airway disease.5, 6, 7
In COPD airway, inflammation is mainly induced by inhaled cigarette smoke or other noxious particles. The pathological changes are found in the airways, lung parenchyma, and pulmonary vasculature. Increased numbers of different cells such as neutrophils, macrophages, and CD8+ T cells and a wide variety of inflammatory mediators have been reported.8 In addition, further characteristics of the disease are manifold structural changes in the lung, ranging from fibrosis around the small airways to loss of lung parenchyma, which results in a rarefication and instability of the small airways.9 There is increasing evidence that in COPD the airway epithelium has an important role in orchestration of inflammatory and immune responses. In addition, epithelial function seems to have a key function in tissue remodeling.10
Unraveling the underlying immunological processes is important, not only to understand the disease but also for the development of novel therapeutic approaches. Indeed, for asthma novel antibodies haven been developed to target Th2-induced inflammation and several of these antibodies are close to be approved for clinical treatment.11 Therefore, research to further elucidate the underlying mechanisms of airway diseases is necessary. Discovered over 30 years ago, Wnt-dependent effects were mainly described during embryogenesis of model organisms such as sea urchin, Drosophila, and Xenopus. Using recombinant ligands, specific inhibitors, and also transgenic mouse models, it is now clear that Wnt pathways are not only involved in developmental processes but also have an essential role in repair processes and regulation of immune responses. The present review summarizes the current knowledge about the role and function of Wnt pathways on immunological and structural changes observed in asthma and COPD.
WNT PATHWAYS
Wnt proteins are a diverse family of secreted lipid-modified cysteine-rich proteins and this structure has made a detailed characterization for a long time almost impossible. In 2003, Wnt3a was purified and the hydrophobic structure common for Wnt proteins was identified.12, 13 Functionally, because of their biochemical properties, Wnt ligands are short-range signaling molecules.14 So far, 19 different Wnt ligands have been identified and structurally described.15 Wnt ligands are recognized by Frizzled (Fzd) receptors, a family of seven-pass transmembrane receptors,16 which are commonly associated with coreceptors such as the LDL receptor-related proteins 5 and 6,17 and also other coreceptors such as ROR or Ryk14 are described. So far, 10 human Fzd genes have been discovered. Ligation of the trimeric Wnt/Fzd/coreceptor complex results in cell signaling. Depending on ligand, receptor, and coreceptor composition, different downstream pathways can be activated. These pathways can be divided into a canonical pathway, now referred to as Wnt/β-catenin, and two noncanonical pathways, which are independent of β-catenin (the planar cell polarity (PCP) and the Wnt-Ca2+ pathway).14 However, cellular context, receptor equipment, and present Wnt ligands can change the normally preferred pathway and induce variations leading to a different unexpected outcome.15
In the current understanding, Wnt1 and Wnt3a ligands act as activators of the Wnt/β-catenin pathway, whereas Wnt5a and Wnt11 are perceived as examples for noncanonical pathway effects.18 The Wnt/β-catenin pathway has been well characterized by its strong dependency on β-catenin. Under steady-state conditions, cytoplasmatic β-catenin is degraded by the proteasome, a process that is strongly controlled by a glycogen synthase kinase-3β (GSK-3β) involving destruction complex. Upon Wnt ligand receptor/coreceptor (LRP5/6) binding, this complex becomes inactivated, leading to cytoplasmic accumulation and subsequent nuclear translocation of β-catenin. In the nucleus, it forms together with T-cell factor and lymphoid enhancer-binding factor, a transcription complex, able to regulate the expression of target genes.18
The noncanonical pathways are completely independent of β-catenin-mediated gene expression. The nomenclature is so far not consistent. Indeed, different defined pathways, such as PCP and Wnt5A-ROR, demonstrate comparable signal cascade and could therefore be regarded as virtually identical.19, 20, 21 The PCP pathway is involved in developmental processes such as regulation of cell polarity, movement, and orientation. This pathway signals via small GTPases such as RHOA and RAC1 and the JUN-N-terminal kinase leading to the activation of JUN-N-terminal kinase depending transcription factors such as ATF2.22 Interestingly, the Wnt/β-catenin and PCP pathways seem to have opposed regulation so that activation of one pathway will suppress the other.23 Wnt5a and Wnt11 are also regarded as prototype ligands for the Wnt-Ca2+ pathway. In the presence of different Fzd receptors, they are able to induce a calcium-dependent signaling cascade via phospholipase C and inositol trisphosphate activation.24, 25, 26, 27
CELL POPULATIONS INVOLVED IN AIRWAY INFLAMMATION
In allergic asthma, several cell types are involved in the inflammatory reaction towards harmless antigens. In healthy subjects, inhaled exposure to an antigen induces tolerance and prevents the development of sensitization towards this harmless molecule. In contrast, under certain circumstances such as the presence of certain danger signals, an orchestrated immune response develops including cells forming the innate (eg, macrophages, DCs, mast cells, innate lymphocytes) and the adaptive immune systems (T cells), and also of structural cells (eg, epithelial cells), which results in the development of specific T- and B-cell responses against the allergen.28, 29, 30, 31 In these sensitized individuals, exposure with the allergen results in specific immunological responses in the lung, in which mast cells and DCs modulate the adaptive immune responses, which is characterized by a Th2 response, including IL-4, IL-5, and IL-13, and accumulation of eosinophilic granulocytes in the airway wall and lumen.32, 33, 34, 35 If these immune responses persist in the airway wall, structural changes, such as goblet cell metaplasia, basement membrane thickening, hypertrophy of ASM, and subepithelial fibrosis, can occur and these structural changes are summarized under the term airway remodeling.2
Dendritic Cells
DCs are commonly regarded as ‘the’ professional antigen-presenting cell and are also in the lung essential to induce proper adaptive immune responses against pathogens or tolerance against harmless antigens. Depending on the degree of activation, the intracellular status and the secreted mediator DCs are the important checkpoint of either activating or suppressing the immune response. Human as well as murine DCs express a variety of Wnt receptors and are so susceptible for canonical as well as noncanonical Wnt signaling.36 Exposition to Wnt ligands and activation of the canonical pathway has been linked to DC-mediated induction of tolerance.37 Interestingly, exposition to Wnt does not lead to a change in the expression of activation markers on DCs such as CD80, CD86, CCR7, or MHCII.37 Still, in vitro exposure of isolated DCs with either Wnt3a (Wnt/β-catenin ligand) or Wnt5a (noncanonical ligand) resulted in the production of different anti-inflammatory mediators, with Wnt3a inducing TGF-β expression, whereas Wnt5a induce the additional expression of IL-10.38 Furthermore, coculture of Wnt-treated DC with naïve T cells resulted in the induction of FoxP3-expressing regulatory T cells38 and also exposure to Wnt1 markedly reduced the activation of effector T cells by DCs in coculture experiments.39 Similar results were observed in a human cell coculture system. Exposure to Wnt5a resulted in increased secretion of IL-10 in blood-derived monocytes.40 DCs exposed to this ligand express more indolamin-2,3-dioxygenase, a tryptophan-degrading enzyme with immune regulatory properties, and induce more regulatory T cells.41
Taken together, these data strongly suggest that Wnt/β-catenin as well as noncanonical Wnt ligands have the ability to shape DCs to a tolerogenic status and thus preventing or regulating adaptive T-cell responses. This is supported by in vivo studies; a murine model of chronic inflammatory bowel disease showed that the expression of β-catenin in DCs is important for the production of anti-inflammatory mediators such as retinoic acid-metabolizing enzymes, IL-10, and transforming growth factor-β.42 In addition, T cells isolated from small and large intestine of animals, which lacked the expression of β-catenin in DCs, demonstrated an increased inflammatory phenotype, and isolated DCs showed a reduced ability to induce a Foxp3-expressing regulatory T cells but an enhanced capacity to induce IL-17 and IFN-γ-expressing effector cells. These data demonstrated for the first time that Wnt signaling, and especially the Wnt/β-catenin pathway, has an important role in the induction/maintenance of a tolerogenic DC phenotype in vivo. Interestingly, the Wnt/β-catenin signaling pathway seems to be also important for other autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE), a murine model for multiple sclerosis.43 Indeed, mice that lacked the expression of the Wnt coreceptors LRP-5/-6 on DCs demonstrated an increased EAE phenotype. Moreover, cells from those animals fail to induce IL-10 production and demonstrate increased secretion of IL-17 and IFN-γ. Furthermore, mice that express active β-catenin or are treated with a β-catenin agonist were more protected from developing EAE associated with reduced IL-17 and IFN-γ production and increased IL-10 expression. Indeed, these results strongly suggest that Wnt/β-catenin-dependent signaling has an immune-suppressive function in vivo. However, if these findings in the gut or the brain could be extrapolated to different organ systems such as the lung was unclear.
T Cells
T cells are the central effector cells of the adaptive immune system. Controlling humoral and cellular responses, they represent long-lasting effective defense mechanisms against viral and bacterial infections. However, T cells also have an important role in autoimmunity and hypersensitivity responses. Indeed, allergen-specific CD4+ T-helper cells are responsible for induction, maintenance, and chronicity of diseases such as allergic asthma. Moreover, induction of different subsets of T cells expressing specific cytokine profiles is discussed as the main reason for the development of heterogeneous asthma phenotypes.2 Induced by DCs, effector T-cell responses are mainly controlled by regulatory T cells (Tregs). These cells are important for suppression of adaptive immune responses and thus for the clearance of immune responses by acting via direct cell contact-dependent or indirect cytokine-dependent mechanisms.44, 45 Moreover, because of the modulation of DC responses, they can also control the development of an adaptive immune response. So far, there are several in vitro studies demonstrating an immune-suppressive effect of Wnt signaling, which is mediated by a direct suppression of effector cells and stabilization of Tregs. Indeed, stabilization of β-catenin in murine Treg supports their survival and their suppressive capacity.46 In addition, stable β-catenin expression in effector T cells induces anergy and prevents inflammatory responses.46 Treatment of polyclonal stimulated human T cells with either Wnt3a or a GSK-3β inhibitor prevented polarization of these cells.47 Moreover, blocking of GSK-3β reduces proliferation and cytokine secretion. These effects could also be observed in cytotoxic CD8+ effector cells.48 Still, Wnt signaling seems to be involved in T-cell homeostasis, as prevention of β-catenin degradation results in lymphopenia, which is mediated by spontaneous activation of naïve T cells in the periphery.49 Taken together, these data demonstrate direct interactions of Wnt ligands with T cells. Especially, T-cell survival and polarization are affected by Wnt ligands and most of the studies suggest that exposure to Wnt ligands induces a suppressive T-cell phenotype.
Macrophages
Macrophages represent one of the first defensive lines of the immune system and are important for the appropriate induction of innate immune responses. Owing to their variety on proinflammatory mediators, they have an important role in the defense against invading pathogenic organisms. However, to prevent harmful effects from an excessive immune response, a well-orchestrated interplay of inflammatory and regulatory mechanisms is essential. Similar to DCs, Wnt signaling has heterogeneous functions in macrophages, which seem to be dependent on the phase of an inflammatory response. Indeed, under homeostatic conditions Wnt5a is necessary to prolong survival of macrophages and keeping the network for immediate anti-microbial immune responses intact.50 In contrast, during acute inflammation Wnt5a is also involved in inducing anti-inflammatory effects from macrophages to prevent an excessive and harmful immune response.50, 51, 52, 53 These data imply that Wnt signaling is important to avoid overwhelming harmful inflammatory reactions in responses to acute infections. In contrast, Wnt ligands have shown no effect on the differentiation into alternative activated M2 macrophages. M2 macrophages themselves have been described to produce Wnt ligands and by this pathway could support the development of a tolerogenic milieu.54 Therefore, comparable to findings in Wnt signaling in DCs, activation of the Wnt pathways in macrophages lead to suppressive responses, especially during inflammatory reactions, thereby preventing excessive inflammation and potentially supporting resolution of inflammation. Again, the immunosuppressive properties of Wnt ligands seem to be strongly context-dependent, as it has been demonstrated that Wnt3a enhances the secretion of TNF-α in an LPS-activated macrophage cell line.55
IMMUNOLOGICAL ROLE OF WNT PATHWAYS IN ASTHMA
In contrast to the well-identified function of different Wnt pathways in embryogenesis and structural development, there is scarce data on the role and function of Wnt expression in airway diseases. Several in vitro studies have demonstrated that Wnt ligands can modulate functions of DCs, T cells, and macrophages, suggesting that Wnt ligands could have a profound impact on innate and adaptive immune responses. As discussed above, pro- and anti-inflammatory properties of Wnt ligands were observed depending on cell type, Wnt ligand, and experimental setup. As mentioned above, evidence from models of inflammation in the brain or gut has already suggested that Wnt ligands display immune-suppressive functions in vivo. However, if these findings could be extrapolated to different organ systems such as the lung was unclear. Interestingly, in human gene expression studies, expression of several Wnt-associated molecules, such as Wnt1-inducible factor signaling pathway protein-1 and Wnt inhibitory factor-1, were associated with impaired lung function.56 In addition, expression of Wnt3a, Wnt5a, Wnt6, Wnt10, and Fzd5 were correlated with a Th2 signature in human asthmatic airways.57 However, these studies describe an association without elucidating the pro- or anti-inflammatory function of these ligands. Recent studies investigated the role and function of Wnt in the context of allergic airway inflammation using an inducible lung-specific over expression of Wnt1. Indeed, induction of Wnt1 before and during airway challenges reduced airway inflammation and airway hyperreactivity, both hallmarks of allergic asthma.39 These effects were detectable in prophylactic situation (increased Wnt expression before the first development of allergic airway disease) and also in a therapeutic context (increased Wnt expression in developed airway disease). Further analysis of the immunological responses showed no change in the proportion of regulatory T cells in lymph nodes or in lung tissue. Instead, the suppressive effects of Wnt1 were related to suppressed migration of antigen-loaded DCs form the lung to the draining lymph nodes and their activation state. Indeed, application of in vitro generated allergen-exposed DCs form wild-type animals was effective in overcoming Wnt1-induced suppression of allergic airway disease. Overall, these results suggest that Wnt1 prevents the activation of pulmonary DCs and by this pathway suppress the development and aggravation of allergic airway disease.
It has been well described that the chemical element lithium is strongly associated with the canonical Wnt pathway. Similar to the effect of canonical Wnt ligands also lithium chloride (LiCl) effectively suppresses the β-catenin destruction complex by inhibiting one of its central components GSK-3β.58 Therefore, LiCl has been widely used as a surrogate compound to assess the involvement of the Wnt/β-catenin pathway.59, 60, 61, 62, 63, 64 Interestingly, the current knowledge of the Wnt pathway can now explain clinical observation made even before the Wnt pathways have been described. Early case reports already demonstrated that treatment of patients suffering from chronic schizophrenia and asthma with LiCl not only reduced the manic episodes but also caused a reduction of asthma attacks and the use of asthma-specific medication,65 and other clinical observations showed that stopping a long-lasting LiCl therapy in a patient-induced increased airway hyper-responsiveness and asthma symptoms.66 The important role of GSK-3β-mediated signaling on the development of asthma was also confirmed in a murine model of allergic airway disease. By blocking GSK-3β before antigen challenge by application of a specific GSK-3β inhibitor, airway inflammation and other features of the asthma phenotype were significantly reduced.67 In addition, recent studies using LiCl in a murine model of allergic airway disease showed that treatment of sensitized animals before and during allergen challenge with LiCl reduced airway inflammation, hyperresponsiveness, and mucus production.39 These finding add a mechanistic explanation to the observed effects in humans.
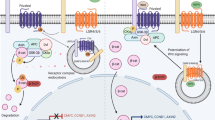
In summary, in allergic asthma the GSK-3β- and β-catenin-dependent canonical Wnt pathway has an immune modulatory function. The immune-suppressive properties of Wnt/β-catenin ligands include the modulation of DC activation, migration, and antigen presentation, and by this pathway prevent induction and secretion of proinflammatory Th2 cytokines and eosinophilic airway inflammation (Figure 1).
Wnt-mediated regulation of allergic airway diseases. Allergic airway disease is driven by misguided immune responses towards a harmless aeroallergen. Besides, dendritic cells (DCs) are central for the induction and exacerbation of an allergic airway disease. DCs take up allergens in the lung, process them, and migrate to the draining lymph node. Here depending on their activation state, they induce anergy or activation of antigen-specific T cells. Once induced, activated effector T cells migrate to the lung and secrete proinflammatory cytokines such as interleukin-13 (IL-13), which lead to the generation of an acute inflammation and structural changes. Wnt1 modulates the migration and phenotype of allergen-loaded DCs. Wnt1-exposed DCs fail to induce an effector T-cell response and therefore also the further features of the disease such as airway inflammation and goblet cell metaplasia.
Role of WNT Pathways in Airway Remodeling
In asthma and COPD not only inflammatory but also structural changes can be found in the airways. Repetitive or persistent exposure of the airway epithelium by allergen contact, air pollutants, cigarette smoke, or cycles of infection leads to chronic inflammation. This epithelial damage can be directly induced by proteolytic activity of allergens,68, 69, 70 histamine,71 or pollen.72 Exposure and stress of the epithelium leads to the production of different cytokines, which induce certain immune responses, and can also be involved in aberrant or uncontrolled tissue repair.73, 74 Remodeling processes in the airways include hypertrophy and migration of ASM, goblet cell metaplasia, basal membrane thickening, subepithelial fibrosis, and airway neovascularization.
Mucus production is one of the features of allergic airway disease. MUC5AC and MUC5B are the principal gel-forming mucins in the lung and are secreted by goblet cells and submucosal glands.75 The molecular mechanisms leading to goblet cell differentiation contain several signaling pathways such as EGFR signaling, STAT6 activation via IL-4 and IL-13, and the Notch signaling pathway. Both EGFR and IL-4 receptor activation lead to the expression of MUC5AC, whereas the activation of Notch signaling is able to inhibit or to induce MUC5AC expression.76 The current hypotheses is a trans-differentiation of existing ciliated and Clara cells to goblet cells, with changes of their form to a more slim and elongated shape. Moreover, the increased number of goblet cells leads to a rise of viscous mucus production, which exacerbates the diminished respiratory airflow of the patients.77 In vivo experiments in mice were able to detect a connection between Wnt/β-catenin signaling and diminished goblet cell metaplasia. Pharmacological inhibition of GSK-3β significantly reduces expression of MUC5AC in a murine model of allergic airway disease.67 In addition, a dose-dependent reduction of IL-13 with rising doses of the GSK-3β inhibitor was detected. In addition, other studies have shown an inhibitory effect of Wnt on goblet cell metaplasia. Both overexpression of Wnt1 in the airways and treatment with LiCl resulted in diminished goblet cell metaplasia.39
Particularly, the increase of smooth muscle mass induced by cellular hyperplasia and hypertrophy is responsible for chronic airflow limitation and increased bronchial hyperresponsiveness. Interestingly, Wnt/β-catenin signaling is involved in proliferation of ASM in vivo and in vitro. Stimulation of ASM with growth factors such as PDGF increases the nuclear β-catenin concentration and is associated with enhanced ASM proliferation, and this effect is dependent on β-catenin.78 Furthermore, β-catenin expression is increased in proliferating human ASM cells.79 Besides these in vitro observations, there are a few in vivo models, which confirm the β-catenin effect on ASM proliferation. On the one hand, Wnt7b/β-catenin signaling is an important factor for ASM development,80 and on the other hand, β-catenin induces proliferation of ASM.81, 82 Moreover, not only proliferation but also contraction of ASM is affected by Wnt/β-catenin signaling. In ASM, β-catenin exists as membrane-associated protein in a complex with actin filament facilitating cell–cell contacts. The activation of Wnt/β-catenin signaling enhances the expression of de novo β-catenin and augmented methacholine-induced contraction.83 These results let us assume that inhibition of Wnt/β-catenin signaling could prevent ASM proliferation and improve AHR.
Many of the insights of the function of Wnt in the context of repair processes in the lung have been identified in models of acute epithelial and lung injury,84, 85, 86, 87 and these studies show an important role for the canonical β-catenin-dependent Wnt pathway during repair processes in the lung. Another mechanism involved in epithelial repair is the so-called epithelial–mesenchymal transition (EMT). EMT is well described in different processes such as organ development, cancer, fibrosis, and tissue repair.88, 89 During EMT epithelial cells differentiate into mesenchymal–fibroblast-like cells. This transition is characterized by reduction of their cell-to-cell contacts, the downregulation of certain markers (such as E-cadherin, claudin, or mucin-1), enhanced cell migration, and induction of mesenchymal gene expression (such as Snail 1, fibronectin, α-smooth muscle actin (α-SMA), vimentin, and cyclin D1).90, 91 Different reports suggest that EMT is linked to airway remodeling in asthma.70, 92 Interestingly, EMT is regulated by the Wnt/β-catenin and the noncanonical Wnt signaling pathway.93, 94, 95 Also in the lung, Wnt signaling is involved in the regulation of EMT. Especially the cross-talk between Wnt/β-catenin signaling and TGFβ, which is a master regulator of EMT, seems to be pivotal in mediating EMT.96, 97 Indeed, a stimulation of cultured cells with an allergen induces β-catenin, especially in the presence of TGF-β. This synergistically leads to a higher expression of the mesenchymal markers fibronectin and vimentin.70 These observations suggest that Wnt/β-catenin and TGF-β are important mediators for the induction of structural changes triggered by EMT in the airways of asthma patients following repeated allergen exposure. Given the so far limited amount of data, further confirmation of these findings would strengthen this concept. Still, the functions of Wnt pathways in mediating EMT are supported by the finding that pharmacological activation of β-catenin in human bronchial cells or overexpression in basal cells upregulates the expression of EMT markers in these cells.85
Function of Wnt Pathways in COPD
In addition, structural changes in the lung of COPD patients seem to be associated with Wnt signaling. One feature of COPD is the development of emphysema with progressive impairment of respiratory functions. Besides, changes in the airways can also be detected, such as mucus hypersecretion by hyperplastic goblet cells and the increased thickening of the wall of small airways.8 Furthermore, EMT is a process involved in remodeling of airways in CODP. Again, Wnt signaling is critically involved in this process. Indeed, Wnt3a and activation of canonical Wnt/β-catenin pathway is upregulated in epithelial cells following nicotine exposure.98, 99 The activation of β-catenin was accompanied by an increase of mesenchymal markers such as α-SMA, vimentin, type 1 collagen, and MMP-9, as well as a downregulation of E-cadherin. The knockdown of Wnt3a prevents the observed effects.100
Another feature of COPD is loss of peripheral lung tissue and the development of emphysema induced by cigarette smoke and environmental factors in people with genetic predisposition.101, 102, 103 Emphysema is characterized by alveolar airspace enlargement, parenchymal destruction, and impaired pulmonary repair.8, 104 Currently, there are no therapeutic agents available, which induces lung regeneration. A potential option for the future could be the reactivation of signaling pathways that are active during lung development to induce epithelial repair in the damaged areas similar to the Wnt/β-catenin pathway.70, 105, 106 In human lung samples of patients with COPD, a reduction of nuclear β-catenin is detectable.64 In addition, decreased Wnt/β-catenin activation has been described in different murine models of emphysema and treatment with LiCl attenuated airspace enlargement and prevents the development of emphysema.64 This protective role of Wnt for the development of emphysema is supported by the finding that Frizzled-related protein-1, an inhibitor of Wnt signaling, induces proteases, which are associated with emphysema formation and tissue destruction.107 If modulating Wnt signaling might pose an interesting therapeutic approach in COPD is under investigation. Very recent experimental findings are promising. In cultures of COPD patient-derived lung tissue, increased Wnt/β-catenin signaling attenuated several pathological features of the cells, suggesting a potential therapeutic effect of increased Wnt signaling.62
CONCLUSION
In summary, recent studies suggest important immune modulatory effects of Wnt molecules on inflammatory as well as structural processes in the airways in asthma and COPD (Figure 2). With regard to inflammatory processes, Wnt pathways mainly seem to elicit regulatory and suppressive functions. Indeed, Wnt/β-catenin and also noncanonical Wnt ligands directly affect DCs, T cells, and macrophages. The exposure to Wnt ligands seems to suppress the development of allergic airway disease and these effects mainly seem to be mediated by effects on DCs. In addition, Wnt ligands are also involved in structural changes in the airway and have been implicated with mucus production and EMT. Interestingly, mucus production seems to be inhibited by Wnt ligands, whereas Wnt ligands seem to be a driver of EMT. Further research is needed to dissect the precise role of the different Wnt ligands in this context. Analyzing the expression of different Wnt ligands, their regulators and changes in the receptor expression in chronic allergen challenge models could help to further understand the dynamics of different Wnt molecules in this setting. In addition, usage of gene-deficient animals as well as long-term overexpression of Wnt ligands in the airways could help to further define the long time effects of these ligands in the remodeling process. The increasing possibilities of targeting these genes in an organ-specific manner using, for example, Cre-recombinase techniques will further help to unravel the pathophysiological function of the different Wnt pathway components. Our increasing knowledge about the effects of Wnt ligands, especially in allergic airway disease or in COPD, could make these pathways an interesting target for pharmacological interventions. Despite these promising results, a careful evaluation is mandatory, given the role and function of Wnt pathways in tumorigenesis.
Impact of Wnt ligands on immune and structural cells. Effects of canonical ligands on different immune and structural cells are displayed in green, whereas effects of noncanonical Wnt ligands are displayed in blue. If similar effects are induced by both canonical and noncanonical ligands effects are displayed as green and blue.
References
Fajt ML, Wenzel SE . Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 2015;135:299–310.
Trejo Bittar HE, Yousem SA, Wenzel SE . Pathobiology of severe asthma. Annu Rev Pathol 2015;10:511–545.
Rayees S, Malik F, Bukhari SI et al. Linking GATA-3 and interleukin-13: implications in asthma. Inflamm Res 2014;63:255–265.
Krug N, Hohlfeld JM, Kirsten AM et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 2015;372:1987–1995.
Watson B, Gauvreau GM . Thymic stromal lymphopoietin: a central regulator of allergic asthma. Expert Opin Ther Targets 2014;18:771–785.
Cianferoni A, Spergel J . The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol 2014;10:1463–1474.
Makrinioti H, Toussaint M, Jackson DJ et al. Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med 2014;2:226–237.
Hogg JC, Timens W . The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009;4:435–459.
McDonough JE, Yuan R, Suzuki M et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567–1575.
Hiemstra PS, McCray PB Jr, Bals R . The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 2015;45:1150–1162.
Olin JT, Wechsler ME . Asthma: pathogenesis and novel drugs for treatment. BMJ 2014;349:g5517.
Willert K, Brown JD, Danenberg E et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003;423:448–452.
Hausmann G, Banziger C, Basler K . Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol 2007;8:331–336.
Niehrs C . The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012;13:767–779.
Kikuchi A, Yamamoto H, Sato A et al. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol 2011;291:21–71.
Logan CY, Nusse R . The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781–810.
He X, Semenov M, Tamai K et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 2004;131:1663–1677.
Staal FJ, Luis TC, Tiemessen MM . WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 2008;8:581–593.
Grumolato L, Liu G, Mong P et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 2010;24:2517–2530.
Ho HY, Susman MW, Bikoff JB et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci USA 2012;109:4044–4051.
Oishi I, Suzuki H, Onishi N et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003;8:645–654.
Veeman MT, Axelrod JD, Moon RT . A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 2003;5:367–377.
Sato A, Yamamoto H, Sakane H et al. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 2010;29:41–54.
Kuhl M, Sheldahl LC, Malbon CC et al. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 2000;275:12701–12711.
Blumenthal A, Ehlers S, Lauber J et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006;108:965–973.
Pereira C, Schaer DJ, Bachli EB et al. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 2008;28:504–510.
De A . Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43:745–756.
Reuter S, Dehzad N, Martin H et al. TLR3 but not TLR7/8 ligand induces allergic sensitization to inhaled allergen. J Immunol 2012;188:5123–5131.
Lambrecht BN, Hammad H . The immunology of asthma. Nat Immunol 2015;16:45–56.
Hammad H, Chieppa M, Perros F et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416.
Yu S, Kim HY, Chang YJ et al. Innate lymphoid cells and asthma. J Allergy Clin Immunol 2014;133:943–950.
Lambrecht BN, Hammad H . The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010;376:835–843.
Reuter S, Dehzad N, Martin H et al. Mast cells induce migration of dendritic cells in a murine model of acute allergic airway disease. Int Arch Allergy Immunol 2010;151:214–222.
Reuter S, Heinz A, Sieren M et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J 2008;31:773–782.
Holgate ST . Innate and adaptive immune responses in asthma. Nat Med 2012;18:673–683.
Valencia J, Martinez VG, Hidalgo L et al. Wnt5a signaling increases IL-12 secretion by human dendritic cells and enhances IFN-gamma production by CD4+ T cells. Immunol Lett 2014;162:188–199.
Jiang A, Bloom O, Ono S et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 2007;27:610–624.
Oderup C, LaJevic M, Butcher EC . Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J Immunol 2013;190:6126–6134.
Reuter S, Martin H, Beckert H et al. The Wnt/beta-catenin pathway attenuates experimental allergic airway disease. J Immunol 2014;193:485–495.
Valencia J, Hernandez-Lopez C, Martinez VG et al. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol 2011;187:4129–4139.
Holtzhausen A, Zhao F, Evans K et al. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol Res 2015;3:1082–1095.
Manicassamy S, Reizis B, Ravindran R et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010;329:849–853.
Suryawanshi A, Manoharan I, Hong Y et al. Canonical wnt signaling in dendritic cells regulates Th1/Th17 responses and suppresses autoimmune neuroinflammation. J Immunol 2015;194:3295–3304.
Bopp T, Dehzad N, Reuter S et al. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol 2009;182:4017–4024.
Taube C, Muller A . The role of Helicobacter pylori infection in the development of allergic asthma. Expert Rev Respir Med 2012:6:441–449.
Ding Y, Shen S, Lino AC et al. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med 2008;14:162–169.
Muralidharan S, Hanley PJ, Liu E et al. Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J Immunol 2011;187:5221–5232.
Gattinoni L, Zhong XS, Palmer DC et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 2009;15:808–813.
Wong C, Chen C, Wu Q et al. A critical role for the regulated wnt-myc pathway in naive T cell survival. J Immunol 2015;194:158–167.
Naskar D, Maiti G, Chakraborty A et al. Wnt5a-Rac1-NF-kappaB homeostatic circuitry sustains innate immune functions in macrophages. J Immunol 2014;192:4386–4397.
Neumann J, Schaale K, Farhat K et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J 2010;24:4599–4612.
Schaale K, Brandenburg J, Kispert A et al. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J Immunol 2013;191:5182–5195.
Bergenfelz C, Medrek C, Ekstrom E et al. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol 2012;188:5448–5458.
Cosin-Roger J, Ortiz-Masia D, Calatayud S et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One 2013;8:e78128.
Kelly JC, Lungchukiet P, Macleod RJ . Extracellular calcium-sensing receptor inhibition of intestinal epithelialTNF signaling requires CaSR-mediated Wnt5a/Ror2 interaction. Front Physiol 2011;2:17.
Sharma S, Tantisira K, Carey V et al. A role for Wnt signaling genes in the pathogenesis of impaired lung function in asthma. Am J Respir Crit Care Med 2010;181:328–336.
Choy DF, Modrek B, Abbas AR et al. Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways. J Immunol 2011;186:1861–1869.
Hedgepeth CM, Conrad LJ, Zhang J et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 1997;185:82–91.
Bentley JK, Deng H, Linn MJ et al. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3(beta) phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol 2009;296:L176–L184.
Deng H, Dokshin GA, Lei J et al. Inhibition of glycogen synthase kinase-3beta is sufficient for airway smooth muscle hypertrophy. J Biol Chem 2008;283:10198–10207.
Du Y, Wang Y, Zhang F et al. Regulation of metastasis of bladder cancer cells through the WNT signaling pathway. Tumour Biol 2015, (in press).
Uhl FE, Vierkotten S, Wagner DE et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J 2015;46:1150–1166.
Rao AS, Kremenevskaja N, Resch J et al. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur J Endocrinol 2005;153:929–938.
Kneidinger N, Yildirim AO, Callegari J et al. Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med 2011;183:723–733.
Nasr SJ, Atkins RW . Coincidental improvement in asthma during lithium treatment. Am J Psychiatry 1977;134:1042–1043.
Convery RP, Hendrick DJ, Bourke SJ . Asthma precipitated by cessation of lithium treatment. Postgrad Med J 1999;75:637–638.
Bao Z, Lim S, Liao W et al. Glycogen synthase kinase-3beta inhibition attenuates asthma in mice. Am J Respir Crit Care Med 2007;176:431–438.
Selman M, Pardo A . The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S93–S97.
Wan H, Winton HL, Soeller C et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 1999;104:123–133.
Heijink IH, Postma DS, Noordhoek JA et al. House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium. Am J Respir Cell Mol Biol 2010;42:69–79.
Zabner J, Winter M, Excoffon KJ et al. Histamine alters E-cadherin cell adhesion to increase human airway epithelial permeability. J Appl Physiol 2003;95:394–401.
Runswick S, Mitchell T, Davies P et al. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007;12:834–842.
Nawijn MC, Hackett TL, Postma DS et al. E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol 2011;32:248–255.
Magnan A, Frachon I, Rain B et al. Transforming growth factor beta in normal human lung: preferential location in bronchial epithelial cells. Thorax 1994;49:789–792.
Voynow JA, Rubin BK . Mucins, mucus, and sputum. Chest 2009;135:505–512.
Boucherat O, Boczkowski J, Jeannotte L et al. Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways. Exp Lung Res 2013;39:207–216.
Lambrecht BN, Hammad H . The airway epithelium in asthma. Nat Med 2012;18:684–692.
Nunes RO, Schmidt M, Dueck G et al. GSK-3/beta-catenin signaling axis in airway smooth muscle: role in mitogenic signaling. Am J Physiol Lung Cell Mol Physiol 2008;294:L1110–L1118.
Gosens R, Baarsma HA, Heijink IH et al. De novo synthesis of {beta}-catenin via H-Ras and MEK regulates airway smooth muscle growth. FASEB J 2010;24:757–768.
Cohen ED, Ihida-Stansbury K, Lu MM et al. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 2009;119:2538–2549.
Volckaert T, Campbell A, De LS . c-Myc regulates proliferation and Fgf10 expression in airway smooth muscle after airway epithelial injury in mouse. PLoS One 2013;8:e71426.
De Langhe SP, Carraro G, Tefft D et al. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One 2008;3:e1516.
Jansen SR, Van Ziel AM, Baarsma HA et al. {beta}-Catenin regulates airway smooth muscle contraction. Am J Physiol Lung Cell Mol Physiol 2010;299:L204–L214.
Zhu M, Tian D, Li J et al. Glycogen synthase kinase 3beta and beta-catenin are involved in the injury and repair of bronchial epithelial cells induced by scratching. Exp Mol Pathol 2007;83:30–38.
Volckaert T, Dill E, Campbell A et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 2011;121:4409–4419.
Liu AR, Liu L, Chen S et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol 2013;228:1270–1283.
Cai SX, Liu AR, Chen S et al. Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 2015;6:65.
Kalluri R, Neilson EG . Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–1784.
Potenta S, Zeisberg E, Kalluri R . The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer 2008;99:1375–1379.
Hackett TL . Epithelial–mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol 2012;12:53–59.
Pain M, Bermudez O, Lacoste P et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev 2014;23:118–130.
Johnson JR, Roos A, Berg T et al. Chronic respiratory aeroallergen exposure in mice induces epithelial–mesenchymal transition in the large airways. PLoS One 2011;6:e16175.
Kim K, Lu Z, Hay ED . Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 2002;26:463–476.
Kato N, Shimmura S, Kawakita T et al. Beta-catenin activation and epithelial–mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci 2007;48:1511–1517.
Dissanayake SK, Wade M, Johnson CE et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 2007;282:17259–17271.
Kim KK, Wei Y, Szekeres C et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 2009;119:213–224.
Kumawat K, Menzen MH, Bos IS et al. Noncanonical WNT-5A signaling regulates TGF-beta-induced extracellular matrix production by airway smooth muscle cells. FASEB J 2013;27:1631–1643.
Krebs M, Sakurai R, Torday JS et al. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res 2010;36:390–398.
Sakurai R, Cerny LM, Torday JS et al. Mechanism for nicotine-induced up-regulation of Wnt signaling in human alveolar interstitial fibroblasts. Exp Lung Res 2011;37:144–154.
Zou W, Zou Y, Zhao Z et al. Nicotine-induced epithelial-mesenchymal transition via Wnt/beta-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2013;304:L199–L209.
Cai Y, Schikowski T, Adam M et al. Cross-sectional associations between air pollution and chronic bronchitis: an ESCAPE meta-analysis across five cohorts. Thorax 2014;69:1005–1014.
Tuder RM, Yoshida T, Arap W et al. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 2006;3:503–510.
Morse D, Rosas IO . Tobacco smoke-induced lung fibrosis and emphysema. Annu Rev Physiol 2014;76:493–513.
Bagdonas E, Raudoniute J, Bruzauskaite I et al. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis 2015;10:995–1013.
Goss AM, Tian Y, Tsukiyama T et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 2009;17:290–298.
Heijink IH, de Bruin HG, Van Den Berge M et al. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax 2013;68:709–716.
Foronjy R, Imai K, Shiomi T et al. The divergent roles of secreted frizzled related protein-1 (SFRP1) in lung morphogenesis and emphysema. Am J Pathol 2010;177:598–607.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Wnt pathways are known to be involved in embryogenesis, as well as lung and cancer development. Recent research has identified a role for Wnt pathways in immune regulation. This review summarizes current understanding of the role and function of different Wnt pathways for allergic airway disease and chronic obstructive pulmonary disease.
Rights and permissions
About this article
Cite this article
Reuter, S., Beckert, H. & Taube, C. Take the Wnt out of the inflammatory sails: modulatory effects of Wnt in airway diseases. Lab Invest 96, 177–185 (2016). https://doi.org/10.1038/labinvest.2015.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.143
This article is cited by
-
Eosinophil accumulation in postnatal lung is specific to the primary septation phase of development
Scientific Reports (2020)
-
Desmoglein 3 Silencing Inhibits Inflammation and Goblet Cell Mucin Secretion in a Mouse Model of Chronic Rhinosinusitis via Disruption of the Wnt/β-Catenin Signaling Pathway
Inflammation (2019)
-
TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/β-catenin signaling
Respiratory Research (2017)
-
TIPE2 Inhibits the Expression of Asthma-Related Inflammatory Factors in Hyperstretched Bronchial Epithelial Cells Through the Wnt/β-Catenin Pathway
Inflammation (2017)