Abstract
The metabolic and hemodynamic alterations in diabetes activate podocytes to increase extracellular matrix (ECM) production, leading to thickening of the glomerular basement membrane (GBM). We hypothesized that diabetes would activate parietal epithelial cells (PECs) in a similar manner and cause thickening of Bowman’s capsules. Periodic acid Schiff staining of human kidney biopsies of 30 patients with diabetic nephropathy (DN) revealed a significantly thicker Bowman’s capsule as compared with 20 non-diabetic controls. The average thickness was 4.55±0.21 μm in the group of patients with DN compared with 2.92±0.21 μm in the group of non-diabetic controls (P<0.001). Transmission electron microscopy confirmed this finding. In vitro, short-term exposure of human PECs to hyperglycemic conditions (30 mM glucose) advanced glycation end products (100 μg/ml) or transforming growth factor-β1 (TGF-β1; 5 ng/ml) increased the mRNA expression of collagen type I α-1, collagen type IV (all six α-chains), bamacan, nidogen 1, laminin α-1, and perlecan. Western blot and colorimetric collagen assays confirmed these results for collagen type IV at the protein level. The production and secretion of TGF-β1 as a possible positive feedback loop was excluded as a mechanism for the autocrine activation of human PECs. To validate these findings in vivo, activation of the PECs was assessed by immunohistochemical staining for CD44 of 12 human biopsy cases with DN. Thickening of the Bowman’s capsule showed strong association with CD44-positive PECs. In summary, metabolic alterations in diabetes activate PECs to increase the expression and secretion of Bowman’s capsule proteins. This process may contribute to the thickening of the Bowman’s capsule, similar to the thickening of the GBM that is driven by activated podocytes. These data may also imply that activated PECs contribute to ECM production once they migrate to the glomerular tuft, a process resulting in glomerular scaring, for example, in diabetic glomerulosclerosis.
Similar content being viewed by others
Main
Diabetic nephropathy (DN) is the world’s leading cause of end-stage renal disease.1 Clinically, DN presents in five stages, leading to a decline in the glomerular filtration rate and an incline in albuminuria over a duration of about 25 years.2 Histomorphologically, DN can be graded in the following four stages: (I) thickening of the glomerular basement membrane (GBM), (II) mesangial expansion, (III) nodular glomerulosclerosis, and (IV) global glomerulosclerosis.3 The pathophysiological mechanisms that feed into DN are numerous and all start from hyperglycemia. As there is no difference in the histomorphological appearance between type 1 diabetes and type 2 diabetes, insulin is probably not a relevant factor contributing to DN. Hyperglycemia, however, triggers endothelial dysfunction and reactive oxygen species production through activation of protein kinase C as an early event.4 Endothelial dysfunction involves decreased production of nitrogen monoxide and increased expression of endothelin-1 and vascular endothelial growth factor.4 Hyperglycemia has direct effects on the production and secretion of extracellular matrix (ECM) proteins by mesangial cells,5, 6 especially on overexpression of the glucose transporters GLUT 1 and GLUT 4.6 The role of podocytes in thickening the GBM in DN has been previously studied.7, 8 Several compounds and pathways have been shown to be important when podocytes thicken the GBM as endothelin-1, transforming growth factor-β (TGF-β), or mTORC1 activation.9, 10, 11
Recently, the previously neglected parietal epithelial cell (PEC) has attracted more attention in diabetic and non-diabetic glomerulosclerosis for its capacity to contribute to either glomerular regeneration or scaring.12, 13, 14 Currently, nothing is known about the role of PECs in glomerular ECM production in diabetic glomerulosclerosis, however, PEC activation and occasional hyperplasia seem to occur.15 We hypothesized that the metabolic alterations in diabetes that activate podocyte-mediated GBM thickening also activate PECs to increase ECM production and that this process leads to thickening of the Bowman’s capsule.
MATERIALS AND METHODS
In vitro Studies
Human PECs were isolated from human kidneys of patients who underwent nephrectomy for renal tumors. The cortices of the kidneys were minced and digested with collagenase IV (750 U/ml; Sigma) for 45 min. Using high-gradient magnetic cell sorting with LS columns (Miltenyi), the cell suspension was depleted of leukocytes (anti-CD45 MicroBeads; Miltenyi). To obtain cells positive for CD133 and CD24, the cells were treated with a second magnetic separation for CD133 (CD133 Cell Isolation Kit, containing the anti-CD133/1 mAb, clone AC133, also used for hematopoietic stem cell sorting). Flow cytometric analysis as previously described16 revealed that the cell suspension positive for CD133 and CD24 was more than 98% pure.17 The cells were cultured in EGM-MV (Lonza, Walkersville, MD, USA) containing 20% FBS (HyClone Laboratories, South Logan, UT, USA). When 90% confluent, the cells were trypsinized, counted, and 1 × 106 cells/well were seeded in 12-well plates for stimulation in EGM-MV containing 5% FBS. The cells were treated for 2 days with VRAD medium composed of DMEM-F12 (Sigma) supplemented with 100 nM vitamin D3, 100 μM RA (all-trans retinoic acid) (Sigma), and 10% FBS for podocyte differentiation. For stimulation, 30 mM glucose (Braun Melsungen AG, Melsungen, Germany), 5 ng/ml recombinant human TGF-β1 (R&D Systems, Abingdon, UK), and 100 μg/ml advanced glycation end products (AGE; Calbiochem Merck, Darmstadt, Germany) were used for 6, 24, 48, or 72 h. As isosmotic glucose solution for infusion was used to stimulate the cells, no osmotic control was needed. A microtetrazolium assay was used to determine cell viability (Promega, Mannheim, Germany). Protein levels of human TGF-β1 in the cell culture supernatant were measured using a sandwich ELISA kit (R&D Systems). Additional to the Western blot analysis, collagen quantification was conducted with a Sirius Red collagen assay kit (Biocolor, Carrickfergus, UK). All kits were used following manufacturers’ protocols.
Histology
The archive of the Institute of Pathology at the LMU Munich was searched for biopsies from patients with a diagnosis of DN that met the following inclusion criteria: time of biopsy between 2003 and 2014, the patient was older than 18 years, native kidney, no systemic disease with renal complications, and type 1 diabetes or type 2 diabetes. Indications for renal biopsy were elevated plasma creatinine levels and glomerular proteinuria in nephrotic range. Patient biopsies with DN were staged using the current pathological classification introduced by Tervaert et al.3 Biopsy specimens for the non-diabetic control group were collected from renal post-transplantation patients and selected for adequate tissue according to Banff criteria with normal glomeruli. Indication for transplant kidney biopsy was a recent rise in plasma creatinine levels and additionally an onset of tubular proteinuria in 2 patients (10% of the cases). In both groups, histology neither revealed an inflammatory glomerular disease, nor an immune complex-mediated glomerulonephritis. Finally, kidney biopsies of 30 patients (19 males, 11 females; mean age 64 years) with DN after long-term diabetes (>5 years) and 20 non-diabetic controls (13 males, 7 females; mean age 39.3 years) were included for morphometric, ultrastructural, and immunohistochemical studies. Out of all 50 selected patient biopsies, 6 randomly selected patients with DN and 6 patients in the non-diabetic control group were selected for transmission electron microscopy (TEM) examination. Independent of the selection for TEM, 12 kidney biopsies from patients with DN who were biopsied between 2007 and 2014 were randomly selected for CD44 assessment. An additional six randomly selected biopsies from the non-diabetic controls biopsied between 2007 and 2014 were stained for CD44. Immunostained biopsies taken before 2007 and cases with insufficient amount of unstained paraffin-embedded tissue left over had to be excluded from the assessment for CD44 staining. The patient records were anonymized according to the recommendations of the ethical committee of LMU Munich.
Morphometry and Immunostaining
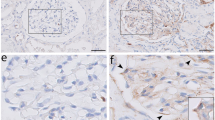
A light microscope (Carl Zeiss, Oberkochen, Germany) was used for the morphometric analysis of periodic acid Schiff (PAS)-stained kidney biopsies as described earlier.18 Measurement of the Bowman’s capsule was performed on 30 biopsies from patients with DN and 20 non-diabetic control biopsies. To exclude thickening artifacts caused by measuring tangentially cut glomeruli, only glomeruli with a diameter of >100 μm were assessed. Under × 400 magnification measurement, lines were drawn from the outer to the inner margin of the Bowman’s capsule in defined distances of 20 μm around the whole glomerulus using the ZEN 2012 imaging software (Carl Zeiss; Figure 1). All measurements were performed by two independent and blinded observers and averages of their results are shown.
Measurement lines were drawn from the outer to the inner margin of the Bowman’s capsule assessed by light- and electron microscope. (a) The measurements of a PAS-stained glomerulus of a patient with DN stage III in the light microscope. (b) The Bowman’s capsule of the PAS-stained slides was measured using a light microscope as displayed in a. All patients in different stages of DN revealed a significant thickened Bowman’s capsule as compared with the non-diabetic controls. The average thickness of the Bowman’s capsule of non-diabetic controls was 2.92±0.21 μm, whereas patients in DN stage I revealed a thickness of 4.45±0.56 μm, patients in DN stage IIa a thickness of 4.86±0.54 μm, patients in DN stage IIb a thickness of 4.66±0.55 μm, patients in DN stage III a thickness of 4.13±0.24 μm, and patients in DN stage IV a thickness of 4.74±0.37 μm. (c) The glomerulus of a non-diabetic control patient in transmission electron microscopy versus (d) a glomerulus of a patient with stage IV DN. Displayed are means with s.e.m. **P<0.01 versus non-diabetic controls; ***P<0.001 versus non-diabetic controls. BC, Bowman’s capsule; DN, diabetic nephropathy; PAS, periodic acid Schiff.
A subset of 12 patient biopsies was further analyzed by TEM. The tissue was fixed in 6.25% glutaraldehyde and 1% OsO4 and embedded in epon. Ultrathin sections, stained with uranyl acetate, were observed under a Zeiss Libra 120 TEM (Libra 120, Carl Zeiss). Gaffney et al.19 considered PECs with ‘round or oval hyperchromatic nucleus’ to be activated. The physiological size of PECs ranges from 0.1–0.3 μm increasing to 2.0–3.5 μm in the perinuclear area.20 Therefore, morphological features of activation were defined as PECs with an enlarged (>3.5 μm) and hyperchromatic nucleus. We performed the measurements of the Bowman’s capsule by drawing lines in distances of 10 μm around the whole glomerulus under × 1250–1600 original magnification using the image SP viewer (SysProg, Minsk, Belarus; Figure 1).
Immunohistochemical staining for CD44 was performed on 3 μm-thick formalin-fixed paraffin-embedded tissue sections. Antigen enhancement and retrieval were done by boiling in ProTaqs VI Antigen Enhancer (quartett Immunodiagnostika, Berlin, Germany) in a microwave for 2 times for 15 min. After washing, sections were blocked with blocking serum from VECTASTAIN Elite ABC-Kit (Universal; Vector Laboratories, Burlingame, CA, USA) and Biotin Blocking System (Dako, Glostrup, Denmark) according to manufacturer’s instructions. Sections were then incubated with anti-CD44 antibody (1:100, mouse monoclonal Ab 156-3C11; Novus Biologicals, Littleton, CO, USA) for 60 min at room temperature. Detection was performed with the VECTASTAIN Elite ABC-Kit (Universal; Vector Laboratories).
Real-Time Quantitative PCR
Total RNA was isolated from the cells using a RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Complementary DNA was generated using reverse transcriptase (Superscript II; Invitrogen, Carlsbad, CA, USA). A SYBR Green Dye detection system was used for quantitative real-time PCR using a Light Cycler 480 (Roche, Mannheim, Germany) with 18S rRNA as a housekeeper gene. Gene-specific primers (Metabion, Martinsried, Germany) were used as listed in Table 1. Controls consisting of ddH2O were negative for target and housekeeper genes. A high confidence algorithm was used to reduce the risk of false-positive crossing points. The melting curve profiles were analyzed for every sample to detect eventual nonspecific products or primer dimers.
Protein Isolation and Western Blot
We extracted protein from renal cells using RIPA buffer (Sigma-Aldrich, St Louis, MO, USA) containing protease inhibitors (Roche, Indianapolis, IN, USA) and processed samples for western blot as described.21 The membranes were incubated overnight at 4°C with rabbit antibody against rat collagen type IV (Novus biologicals, Cambridge, UK). On the following day, membranes were washed, then incubated with peroxidase-conjugated goat anti-rabbit IgG (Cell Signaling Technology, Boston, MA, USA) for 1 h at room temperature. Blots were developed using enhanced chemiluminescence substrate Western Lightning Plus-ECL (Perkin Elmer, Waltham, MA, USA). To quantify collagen type IV expression, western blots were analyzed by digital image analysis software (Image J Gel Analyzer Software, Thermo Fisher Scientific). Data are shown relative to β-actin expression.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 Software (GraphPad Software, La Jolla, CA, USA) and Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). For direct comparisons between two groups, student’s t-test was used with a P value of <0.05 considered statistically significant. All values are expressed as means±s.e.m./s.d.
RESULTS
PEC Activation in DN
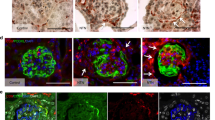
As PECs are the producers of Bowman’s capsule proteins, the Bowman’s capsule thickening in DN could be a result of PEC activation. We used immunohistochemical staining of CD44 for samples from 12 patients with DN and 6 patients in the non-diabetic control group to further confirm the activation of PECs in DN. CD44 is a hyaluronic acid receptor, which is expressed in a large number of human cell types. However, in the glomerular compartment, CD44 is used as an activation marker of PECs and CD44+ PECs seem to contribute to ECM deposition in the glomerular tuft during focal segmental glomerulosclerosis.14, 22 In DN, we found increased expression of CD44 on PECs in association with a significantly increased thickness of the underlying Bowman’s capsule (Figure 2). There was no staining of morphologically inconspicuous PECs, indicating that CD44 is a late expression marker of severely altered glomeruli in DN. In addition to the CD44 staining of the kidney biopsies, TEM was used to investigate ultrastructure of PECs in DN. Although the technical limitations of TEM and small number of PECs with orthogonal sections through the nuclei did not allow a statistical analysis, Figure 2 clearly represents PECs with both signs of activation in a patient with DN. Thus, CD44 staining revealed activation of PECs in DN.
Metabolic alterations in DN activate PECs as displayed by CD44 expression and changes in the ultrastructural morphology of the PEC nuclei. On PECs, CD44 is expressed at late phases of activation and associated with a thickened Bowman’s capsule in a patient with stage IV DN (a) in comparison with a patient in the non-diabetic control group, where PECs do not express CD44 (b) under × 400 magnification. Ultrastructural activation of PECs is displayed as enlarged diameter of the nuclei (>3.5 μm) and euchromatic nuclei assessed by TEM under × 1600 magnification of a patient with stage IIa DN (d) in comparison with a patient in the non-diabetic control group (c). DN, diabetic nephropathy; PECs, parietal epithelial cells; TEM, transmission electron microscopy.
Collagen Expression of PECs Under Diabetic Condition In Vitro
What drives PEC activation and Bowman’s capsule thickening in diabetes? To address this question, we performed in vitro experiments and stimulated human PECs with AGE (100 μg/ml), TGF-β1 (5 ng/ml), or high glucose (30 mmol) for 6, 24, 48, or 72 h. Table 1 lists all the investigated genes of Bowman’s capsule proteins according to the published composition of the Bowman’s capsule.23 Gene expression analysis for all α-chains of collagen type IV and collagen type I α-1, bamacan, laminin α-1, nidogen 1, and perlecan on in vitro stimulation of human PECs showed significant upregulation at different time points (Figure 3). However, we also witnessed downregulation of the α-chains 3 and 4 of collagen type IV after 24 h following stimulation with high glucose and AGEs. Comparison of the different stimuli revealed a higher stimulatory effect of AGEs and glucose at 6 and 24 h, whereas stimulation with TGF-β1 lead to significant upregulations at 6, 24, and 48 h (Figure 3). Taken together, human PECs stimulated with AGEs, TGF-β1, and high glucose increase the mRNA expression of all Bowman’s capsule components and collagen type I α-1 at different time points. Co-stimulation of PECs with differentiation medium (VRAD) and all three stimuli revealed no alteration in the capacity of PECs to express the podocyte marker nephrin (Figure 4).
Stimulation of human PECs with AGEs, TGF-β1, and high glucose upregulates the expression of Bowman’s capsule genes. Following the stimulation of PECs with AGEs (100 μg/ml), TGF-β1 (5 ng/ml), and high glucose (30 mM) for 6, 24, and 48 h, all Bowman’s capsule genes are increasingly expressed at different time points. Significant downregulation of the alpha chains 3 and 4 of collagen type IV was witnessed after 24 h following stimulation with high glucose and AGEs. Displayed are means with s.e.m. *P<0.05 versus control. AGEs, advanced glycation end products; PECs, parietal epithelial cells; TGF-β, transforming growth factor-β.
Stimulation with AGEs, TGF-β1, and high glucose does not upregulate the expression of TGF-β1 in human PECs or alter them to express the podocyte marker nephrin under differentiation conditions. (a) The addition of AGEs (100 μg/ml), TGF-β1 (5 ng/ml), and high glucose (30 mM) to the medium containing VRAD for 48 h does not alter the capacity of PECs to express nephrin. There was no change in the mRNA levels of TGF-β1 following the stimulation at any time point. AGEs, advanced glycation end products; PECs, parietal epithelial cells; TGF-β, transforming growth factor-β.
We first performed the Sircol soluble collagen assay to validate these results at the protein level. In identical experiments, PECs showed significant production and secretion of collagen into the supernatant after 48 h following stimulation with AGEs, TGF-β1, and high glucose (Figure 5). These changes were not detected after stimulation for only 24 h. Analysis for the production of non-secreted collagen that remained within the cells revealed significantly higher levels at 48 h on stimulation with TGF-β1 and AGEs. There was significant downregulation of collagen production after stimulation with TGF-β1 for 24 h.
PECs produce and secrete collagen upon stimulation with AGEs, TGF-β1, and high glucose. Stimulation for only 24 h revealed no changes in collagen secretion into the supernatant (a), whereas significantly less collagen was detected in the cells upon stimulation with TGF-β1 (5 ng/ml) for 24 h (c) as assessed by the collagen assay (Sirius Red collagen assay kit, Biocolor). Following the stimulation for 48 h, the PECs produce and secrete significant higher amounts of collagen (b and d). (e) PECs were stimulated with AGEs (100 μg/ml), TGF-β1 (5 ng/ml), and high glucose (30 mM) for 24, 48, and 72 h. Detection of collagen IV using western blot revealed an upregulation of collagen type IV at all time points following stimulation with either AGEs, TGF-β 1, or high glucose relative to the β-actin expression. Displayed are means with s.e.m. *P<0.05 versus control; **P<0.01 versus control; ***P<0.001 versus control. AGEs, advanced glycation end products; PECs, parietal epithelial cells; TGF-β, transforming growth factor-β.
Furthermore, we also performed western blot to more specifically examine the production of collagen type IV. Parallel experiments using the same settings at 72 h clearly showed an increase in collagen type IV protein production on stimulation with AGEs and high glucose, and especially with TGF-β1 stimulation. The effects of upregulation of collagen type IV on stimulation were detectable after 24 h and continued to increase to a maximum effect at 72 h (Figure 5). In summary, these results confirmed the findings for mRNA and showed production and secretion of collagen on stimulation after 48 and 72 h.
These in vitro data validate that hyperglycemic conditions, AGEs and TGF-β1 activate PECs to upregulate collagen production and secretion into extracellular compartments, which for PECs implies into the Bowman’s capsule as well as into the glomerular filtrate.
Production and Secretion of TGF-β1 by PECs
Do PECs produce TGF-β1 and activate themselves via an autocrine pathway? To answer this question, we examined the expression of TGF-β1 following stimulation with AGEs, TGF-β1, and high glucose for 6, 24, and 48 h. On an mRNA level as well as on a protein level (as investigated by ELISA, data not shown), there was no upregulation of TGF-β1. These results exclude a TGF-β1-based autocrine activation loop for PECs and imply that TGFR activation in PECs requires extrinsic sources of TGF-β1 release.
Bowman’s Capsule Thickness in Biopsies from Diabetic and Non-Diabetic Patients
We identified 30 patients with DN and 20 non-diabetic control patients being biopsied in the time frame 2003–2014. Staging all biopsies in the pathological classification by Taevert,3 four patients were graded stage I, seven patients graded stage IIa, eight patients graded stage IIb, eight patients graded stage III, and three patients graded stage IV. In the group of patient biopsies with DN, 9.6 glomeruli within average 30.6 measurements could be assessed as in comparison with the group of non-diabetic controls with 8.1 glomeruli and 32.9 measurements. All patients with DN had a significant thicker Bowman’s capsule in comparison with the non-diabetic control patients with a mean thickness of 4.55±0.21 μm. Measurements of the non-diabetic control biopsies revealed a mean thickness of 2.92±0.21 μm. There was no significant difference in the Bowman’s capsule thickness among the different stages of DN (Figure 1). Thus, DN involves thickening of the Bowman’s capsule.
To validate these results, a further subset of 12 kidney biopsies for which TEM samples were available were evaluated. Here, in average, 150 measurement lines per glomerulus of the Bowman’s capsule were drawn. The measurements revealed a mean Bowman’s capsule thickness of 4.2±0.88 μm in the patients with DN versus 2.8±0.48 μm in the non-diabetic control group, thus confirming a thickening of the Bowman’s capsule in patients with DN.
Discussion
Our results show that metabolic alterations in diabetes are sufficient to activate an increase in ECM production in PECs leading to a thickening of the Bowman’s capsule. We approached the hypothesis by in vitro experiments with human PECs and morphometrical analysis of renal biopsies of patients with DN. One study from Gaffney et al.19 had shown a ‘prominent parietal epithelium’ associated with significant thickening of the Bowman’s capsule in a subset of five patients with DN. Although there is little known about the changes of the parietal layer of the glomerular capsule in DN, thickening of the visceral layer of the glomerular capsule is already part of the classification of the different stages of DN.3 Our morphometric analysis of the Bowman’s capsule in PAS-stained slides (at an average of almost 10 glomeruli per biopsy) clearly confirmed the findings by Gaffney et al. in 30 patients with different stages of DN and 20 non-diabetic control patients. The TEM results confirmed the thickening of the Bowman’s capsule in a subset of 12 patients. There was no correlation between the stages of DN and the thickness of the Bowman’s capsule as it could be found in a study correlating the width of the GBM with the degree of albuminuria in patients with T2D.24 As there is already thickening of the Bowman’s capsule in patients with DN stage I, the thickening of the Bowman’s capsule in DN seems to represent early changes in DN. This can be explained by an early exposure of the PECs to metabolic changes, as glucose and partly AGEs25 are freely filtered through the GBM-affecting PECs.
As there is also significant hyperfiltration in patients with DN that could contribute to the thickening of the Bowman’s capsule, we used in vitro studies to show the relevance of the metabolic alterations for the thickening of the Bowman’s capsule. They revealed increased expression of all α-chains of collagen type IV, of collagen type I α-1, bamacan, laminin α-1, nidogen 1, and perlecan on stimulation with AGEs, TGF-β1, or high glucose at different time points (Figure 3). Nevertheless, there was also a slight downregulation of collagen type IV α-3 and -4 mRNA. These results are in agreement with published literature, where an upregulation of collagen type I α-1 and collagen type IV α-1 and α-3 on stimulation with TGF-β1 was already known.26 Previous studies using in situ hybridization also had shown increased mRNA levels of TGF-β1 and collagen type IV in PECs in patients with DN.27 Both collagen and western blot assays showed an increased production and secretion of collagen type IV protein. Again, this was confirmatory to the published increased production of collagen type IV on high-glucose treatment in glomerular epithelial cells in vitro28 and explains the thickening of the Bowman’s capsule in patients with DN. Those in vitro data confirm the histological finding of activated PECs, which thicken the Bowman’s capsule in patients with DN similar to the thickening of the GBM by activated podocytes. Former studies had shown increased mRNA levels of TGF-β1,27 wherefore the production of TGF-β1 partly was focus of our studies. Here, no upregulated production and secretion of TGF-β1 was detected either at mRNA or at protein level. In our study, exposure of PECs to TGF-β1 increases thickening of the Bowman’s capsule and could contribute to glomerulosclerosis, but there is no production of TGF-β1 by PECs themselves under diabetic condition in vitro excluding a possible autocrine feedback mechanism. As limitation to the in vitro studies, a short duration of stimulation (maximum 72 h) was chosen to avoid additional formation of AGEs in the high-glucose treatment group. This, however, is far from the condition of patients suffering from type 1 diabetes or type 2 diabetes for several years until development of DN. Another limitation is the use of human PECs that were isolated for their CD24 and CD133 positivity and represent just a small progenitor subset of all PECs. In embryonic kidney differentiation, these stem cell markers are exhibited on a high number of cells in the early phase (35–50% positivity for CD24 and CD133 of a total kidney cell suspension at gestational age of 8.5–9 weeks). Through maturation, the proportion of CD24+CD133+ decreases until 0.5–3% of a total kidney cell suspension in adult human kidneys. In human embryonic kidneys, CD24 is mainly expressed on structures deriving from the metanephrogenic mesenchyme and in the ureteric bud, whereas CD133 expression is to be found on primary vesicles, comma-shaped bodies, and S-shaped bodies. CD133 expression is restricted to a subset of CD24+ cells. In adult human glomeruli, CD133 and CD24 are selectively maintained on PECs in proximity to the urinary pole.16, 29 This subset of PECs was named renal progenitor cells as they showed the capacity to differentiate in vitro into tubular cells, osteogenic cells, adipocytes, and also cells that are in phenotype and function similar to neuronal cells.16 Most importantly, those renal progenitor cells showed ability to differentiate to podocytes by using DMEM medium containing Vitamin D3 and retinoic acid.17 The addition of all three stimuli to VRAD medium did not alter the capacity of the CD24+CD133+ cells to express podocyte marker (Figure 4).
In summary, the metabolic alterations in diabetes activate PECs to increase the expression and secretion of ECM proteins. This process could contribute to the thickening of Bowman’s capsule similar to the thickening of the GBM that is driven by activated podocytes. These data may also imply that activated PECs contribute to ECM production once they migrate to the glomerular tuft, a process that could contribute to diabetic glomerulosclerosis.
References
Prakash J, Lodha M, Singh SK et al. Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India 2007;55:412–416.
Mogensen CE, Christensen CK, Vittinghus E . The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983;32 (Suppl 2):64–78.
Tervaert TW, Mooyaart AL, Amann K et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556–563.
Kanwar YS, Wada J, Sun L et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11.
Harris RD, Steffes MW, Bilous RW et al. Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int 1991;40:107–114.
Heilig CW, Concepcion LA, Riser BL et al. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest 1995;96:1802–1814.
Herbach N, Schairer I, Blutke A et al. Diabetic kidney lesions of GIPRdn transgenic mice: podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Am J Physiol Renal Physiol 2009;296:F819–F829.
Ziyadeh FN, Wolf G . Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 2008;4:39–45.
Inoki K, Mori H, Wang J et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 2011;121:2181–2196.
Lee HS . Mechanisms and consequences of TGF-ss overexpression by podocytes in progressive podocyte disease. Cell Tissue Res 2012;347:129–140.
Lenoir O, Milon M, Virsolvy A et al. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol 2014;25:1050–1062.
Pichaiwong W, Hudkins KL, Wietecha T et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 2013;24:1088–1102.
Romagnani P, Remuzzi G . Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 2013;24:13–20.
Smeets B, Kuppe C, Sicking EM et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 2011;22:1262–1274.
Gaut JP, Hoshi M, Jain S et al. Claudin 1 and nephrin label cellular crescents in diabetic glomerulosclerosis. Hum Pathol 2014;45:628–635.
Sagrinati C, Netti GS, Mazzinghi B et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 2006;17:2443–2456.
Ronconi E, Sagrinati C, Angelotti ML et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 2009;20:322–332.
Patole PS, Pawar RD, Lichtnekert J et al. Coactivation of Toll-like receptor-3 and -7 in immune complex glomerulonephritis. J Autoimmun 2007;29:52–59.
Gaffney EF . Prominent parietal epithelium: a common sign of renal glomerular injury. Hum Pathol 1982;13:651–660.
Shankland SJ, Anders HJ, Romagnani P . Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens 2013;22:302–309.
Mulay SR, Thomasova D, Ryu M et al. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int 2012;81:1199–1211.
Fatima H, Moeller MJ, Smeets B et al. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 2012;7:1852–1858.
Miner JH . Renal basement membrane components. Kidney Int 1999;56:2016–2024.
Weil EJ, Lemley KV, Mason CC et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 2012;82:1010–1017.
Turk N, Mornar A, Mrzljak V et al. Urinary excretion of advanced glycation endproducts in patients with type 2 diabetes and various stages of proteinuria. Diabetes Metab 2004;30:187–192.
Smeets B, Angelotti ML, Rizzo P et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 2009;20:2593–2603.
Umezono T, Toyoda M, Kato M et al. Glomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathy. J Nephrol 2006;19:751–757.
Danne T, Spiro MJ, Spiro RG . Effect of high glucose on type IV collagen production by cultured glomerular epithelial, endothelial, and mesangial cells. Diabetes 1993;42:170–177.
Lazzeri E, Crescioli C, Ronconi E et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 2007;18:3128–3138.
Acknowledgements
We thank Anja Heier, Andrea Sendelhofert, and Sabine Schäfer for their expert technical assistance. This project was supported by a grant from the Deutsche Forschungsgemeinschaft (AN372/11-2) to HJA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study investigates thickening of the Bowman's capsule in patients with diabetic nephropathy and unravels its pathomechanism by assessing the production of extracellular matrix proteins by activated parietal epithelial cells under diabetic conditions. The authors conclude that the metabolic changes in diabetes activate parietal epithelial cells and cause thickening of Bowman's capsules.
Rights and permissions
About this article
Cite this article
Holderied, A., Romoli, S., Eberhard, J. et al. Glomerular parietal epithelial cell activation induces collagen secretion and thickening of Bowman’s capsule in diabetes. Lab Invest 95, 273–282 (2015). https://doi.org/10.1038/labinvest.2014.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2014.160
This article is cited by
-
Diabetic condition induces hypertrophy and vacuolization in glomerular parietal epithelial cells
Scientific Reports (2021)
-
Parietal epithelial cell dysfunction in crescentic glomerulonephritis
Cell and Tissue Research (2021)
-
MYDGF attenuates podocyte injury and proteinuria by activating Akt/BAD signal pathway in mice with diabetic kidney disease
Diabetologia (2020)
-
GYY4137, a Hydrogen Sulfide Donor Modulates miR194-Dependent Collagen Realignment in Diabetic Kidney
Scientific Reports (2017)
-
Effect of Linagliptin on Structural Changes in the Kidney in Experimental Type 2 Diabetes Mellitus
Bulletin of Experimental Biology and Medicine (2016)