Abstract
The polycomb complex protein Bmi1 (B lymphoma Mo-MLV insertion region 1 homolog) mediates epigenetic transcriptional silencing by modifying chromatin structure and is critical for stem cell homeostasis and tumorigenesis. Bmi1 is frequently overexpressed in human malignancies and therefore has key diagnostic and prognostic significance, and holds potential as a therapeutic target. Here we sought to characterize the expression patterns and oncogenic roles of Bmi1 in tongue squamous cell carcinoma and to determine the anticancer effects of histone deacetylase inhibitors (HDACis) via Bmi1 inhibition against tongue cancer. Our data revealed that Bmi1 was aberrantly overexpressed in a significant portion of tongue cancers. Elevated Bmi1 is associated with cervical node metastasis, Ki-67 abundance and reduced overall survival, and also serves as an independent prognostic factor for patient outcomes. Short-hairpin RNA-mediated Bmi1 knockdown inhibited cell proliferation and migration, induced cell apoptosis and senescence, reduced colony formation and CD44+CD133+ sub-population as well as enhanced cisplatin chemosensitivity, presumably by modulation of p16, p14 and E-cadherin. Moreover, HDACi chemicals Trichostatin A (TSA) and sodium butyrate (NaB) potently inhibited Bmi1 and triggered similar phenotypic changes reminiscent of Bmi1 silencing, although TSA treatment seemed paradoxically to induce some epithelial–mesenchymal transition-like changes in tongue cancer cells. Importantly, NaB-induced antitumor effects were partially attenuated by enforced Bmi1 overexpression in vitro. Genetic Bmi1 silencing and pharmacological inhibition of Bmi1 by NaB treatment significantly impaired tumor growth in a tongue cancer xenograft model. Taken together, our results indicate that Bmi1 serves as a key driver and biomarker with multiple oncogenic functions underlying tongue tumorigenesis. Selected appropriate HDACi compounds like NaB may represent novel therapeutic agents against tongue cancer.
Similar content being viewed by others
Main
Oral cancer is one of the most common cancers worldwide, approximately accounting for 3% of all malignancies in both sexes. It is widely represented as a heterogeneous tumor with aggressive phenotypes and behaviors. The major etiological risks for this malignancy include smoking and alcohol consumption and human papillomavirus infection.1 The overwhelming majority of oral cancers arises from tongue and is pathologically identified as squamous cell carcinoma (SCC).2 Despite tremendous advancement in multimodal therapies against oral cancers over the past decades, the overall 5-year survival rate with these devastating diseases, especially those with advanced diseases, has not been markedly improved.3 Local relapse and cervical lymph node metastasis are recognized as the most prevalent factors affecting patients’ survival. Although many oncogenes and tumor suppressors have been identified as key factors underlying oral tumorigenesis, however, no optimal and commonly accepted biomarkers have been established to facilitate disease diagnosis, treatment selection and prognostic prediction.4 Thus, these facts reflect the aggressive nature of oral cancer and therapeutic challenge for clinicians. Further identification and verification of new biomarkers and therapeutic targets for oral SCC especially the tongue SCC (TSCC) are paramount and urgent to optimize diagnosis and treatment strategies for this malignancy.
Polycomb group proteins are critical transcriptional repressors that epigenetically modify chromatin and govern cell fate decision, stem cell self-renewal and cancer development.5 B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) is the core member of the polycomb repressive complex 1 (PRC1) required for monoubiquitination of histone 2A, usually functions as an epigenetic silencer of many target genes such as Ink4a-arf locus.6 Bmi1-mediated chromatin silencing has been found to be involved in diverse fundamental cellular processes, such as cell proliferation, apoptosis, senescence, epithelial–mesenchymal transition (EMT) and stem cell maintenance.7, 8 There is increasing evidence that aberrant overexpression of Bmi1 is associated with multiple human malignancies such as myeloid leukemia, lung cancer, colorectal and breast cancer, and head neck cancer. Furthermore, its overexpression often correlates with advanced stages, aggressive clinicopathological behavior, therapeutic resistance and poor prognosis in these cancers.9, 10, 11, 12 Enforced overexpression of Bmi1 promoted malignant transformation, cancer cell proliferation, EMT and metastatic spreading, whereas its depletion inhibited cell proliferation, migration and invasion and induced cell apoptosis and senescence both in vitro and in vivo.13, 14, 15 Noticeably, Bmi1 has critical and indispensable roles in maintaining the self-renewal capacity of normal and malignant cancer stem cells, which the latter is increasingly recognized to largely account for cancer initiation and progression, therapeutic resistance and disease relapse.7, 16, 17 A myriad of direct or indirect target genes including p16, p14 and E-cadherin are partially responsible for the essential oncogenic roles of Bmi1 in diverse cancer contexts.6, 8, 18 These findings have firmly established that Bmi1 is an important bona fide driver with pleiotropic oncogenic roles during tumorigenesis, thus leading to the hypothesis that blocking Bmi1 expression/activity and its downstream signaling cascade represents a promising strategy for novel anticancer treatment. Indeed, several reports have shown that genetic silencing and pharmacologic inhibition of Bmi1 induced cell proliferation arrest, apoptosis, senescence, and increased susceptibility to chemotherapeutic agents, ultimately impaired cancer progression.19 More recently, Bmi1 has been identified as an indispensable factor to maintain the tumor-seeding abilities of colorectal cancer-initiating cells and targeting Bmi1 with a small-molecule inhibitor resulted in colorectal cancer-initiating cells loss with long-term and irreversible impairment of tumor growth.20 Thus, these findings support the notion that targeting Bmi1 may be an attractive and plausible way to eradicate cancers as a novel therapeutic strategy.
Recently, the histone deacetylase inhibitors (HDACis) have attracted much attention as novel and promising epigenetic therapeutic agents, mainly due to their capacities to induce growth inhibition, cell apoptosis and premature senescence, thereby achieving significant anticancer effects in multiple cancer cells and preclinical animal models.21 The primary targets of HDACi are histone deacetylases with varied potency and specificity, however, the detailed mechanisms behind remain incompletely known and probably involve multiple molecular targets depending on cellular and developmental contexts.22 Intriguingly, Bmi1 has been identified as a novel transcriptional target of HDACi and was successfully inhibited in human breast cancer and acute myeloid leukemia cells via pharmacological approach. Three canonical HDACi chemical compounds including sodium butyrate (NaB), valproic acid and Trichostatin A (TSA) have capacities to downregulate Bmi1 and derepressed its downstream targets, which are known to be silenced by Bmi1.23, 24, 25 Collectively, these results raise the possibility that therapeutic targeting Bmi1 by HDACi may hold potentials as a novel anticancer strategy when used as single agent or in combination with current therapeutic agents.
Accumulating evidence has indicated that Bmi1 serves as an essential regulator during the initiation and progression of head neck cancers.26, 27, 28 Overexpression of Bmi1 significantly associated with malignant transformation of oral precancerous lesions, EMT of oral epithelial cells and cancer stem cell maintenance.29, 30 Moreover, elevated Bmi1 correlated with aggressive features and unfavorable patients’ survival.9, 26, 27 However, the in-depth investigations into the expression pattern of Bmi1 and associated molecular mechanisms underlying TSCC development remain to be further clarified. Intriguingly, therapeutic efficiency of targeting Bmi1 by HDACi compounds in tongue cancer cells remains unexplored. Therefore, in this study, we first examined the Bmi1 expression and its clinicopathological significance in tongue cancer cell lines and clinical samples. Then, the biological roles responsible for Bmi1 in tongue cancer progression were identified by loss-of-function assays via both short-hairpin RNA (shRNA)-mediated genetic and HDACi-mediated pharmacological approaches in vitro and in vivo.
MATERIALS AND METHODS
Tongue Cancer Cell Lines and Chemical Reagents
A panel of human TSCC cell lines HN4, HN6, HN12, Tca8113, Cal27, SCC9 and SCC25 were used here. The HN cells were obtained from National Institutes of Health. Tca8113 was previously established at Shanghai Jiaotong University (a generous gift from Professor Wantao Chen).31 The Cal27, SCC9 and SCC25 cells were purchased from American Type Culture Collection (ATCC). Cancerous cell lines were grown in DMEM (Invitrogen) supplemented with 10% FBS (Gibco) and 100 units/ml penicillin and streptomycin, and maintained in a humidified incubator with 5% CO2 at 37 °C. The HDACis TSA and NaB were purchased from Sigma Aldrich (category number: T8552, B5887) and dissolved in DMSO or PBS as stocking solutions and diluted with culture medium upon addition into cells. Cells were treated with diverse concentrations of TSA or NaB for the indicated times. For chemotherapy sensitivity assay in vitro, shRNA-mediated Bmi1-silencing cells were further treated with cisplatin (Sigma Aldrich, category number: P4394, 2.5 μg/ml) or these cancerous cells were exposed with cisplatin alone or in combination with TSA or NaB for the indicated times. For in vitro cellular rescue experiments, following pretreated with NaB (2 μM) for 48 h, the cells were further transiently transfected with pcDNA3-Bmi1 plasmid (gifted from Professor Muh-Hwa Yang, National Yang-Ming University, Taiwan)8 for another 48 h and then subjected to further experiments.
Bmi1 shRNA Plasmids Construction and In Vitro Transfection
Two shRNA interference plasmids targeting different regions of human Bmi1 were designed and purchased from Shanghai GenePharma. The efficiency and specificity of these shRNA sequences had been verified previously.8, 32 The knockdown efficiency of each shRNA construct was further confirmed by in vitro delivery to cells using lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendations. After transfection for 24 or 48 h, the cells were harvested for the further experiments.
Cell Immunofluorescence and Imaging
The cancerous cells were seeded and grown on glass coverslips 24 h before experiment. After fixed with 4% paraformaldehyde-PBS for 15 min, cells were then permeabilized in 0.1% Triton X-100 in PBS and sequentially blocked with 3% bovine serum albumin for 30 min. Following the overnight incubation with primary antibodies specific for Bmi1 (Cell Signaling, # 6964, 1:200 dilution), E-cadherin (Cell Signaling, # 3195, 1:250 dilution), these cells were further incubated with appropriate secondary antibodies and cytoskeleton actin staining. Immunofluorescence was visualized under a Zeiss fluorescence microscope and image captured.
RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen) and then reversely transcribed into first strand cDNA using PrimeScriptTM RT reagent kit (Takara). The generated cDNA was used for real-time PCR reaction using SYBR Premix Ex TaqTM kit (Takara) following the supplier’s instructions. The gene-specific primers for human Bmi1, Ezh2, p16, p14, E-cadherin, Vimentin, CD44, CD133 and GAPDH were purchased commercially (Invitrogen). Relative mRNA expression of each gene as compared with internal control GAPDH was quantified using comparative CT method.
Western Blot Analysis
The cells with 80–90% confluence in culture flasks were lysed in ice-cold buffer containing protease inhibitor cocktail (Roche) as we reported before.33 Equal amounts of protein samples were loaded and separated by 8–12% SDS-PAGE and transferred to PVDF membranes (Millipore), which then were blocked by 5% non-fat dry milk. These blots were incubated at 4 °C overnight with primary antibodies Bmi1, EZH2, p16, cleaved-PARP (Cell Signaling, 1:1000 dilution), E-cadherin, Vimentin (BD Biosciences, 1:2000 dilution) and GAPDH (Santa Cruz, 1:2000 dilution) followed by incubations with secondary antibodies. The relative levels of each protein were quantified with Quantity One software (Bio-Rad).
MTT Assay
Cell proliferation and viability were monitored by absorbance using MTT assay. Approximately 1000–3000 cells per well were seeded in the 96-well plates. At the indicated time-points, 5 mg/ml MTT (Sigma) was added to the cells and incubated at 37 °C for another 4 h. Absorbance at 490 nm was measured with an automatic enzyme-linked immunosorbent assay reader (BioTek Instruments).
Flow Cytometry Analysis
Cells were treated with trypsin and resuspended as single-cell suspension. For apoptosis assay, cells were stained with Annexin V: PE Apoptosis Detection Kit (BD Bioscience). For cell surface maker assay and cell sorting, cells were labeled with human fluorochrome-conjugated anti-CD44-APC (BD Pharmingen) and anti-CD133-PE (Miltenyi Biotec) and then sorted by BD FACSVantage flow cytometer. The corresponding immunoglobulins conjugated antibodies were used as isotype controls in each experiment.
In Vitro Cell Migration and Wound-Healing Assay
Cell migration assay was performed using chambers (8-μm pore size, Corning) in 24-well plates. Forty-eight hours after shRNA transfection, cells were detached and resuspended, then seeded into the upper chambers with medium containing 1% FBS. Complete medium containing 10% FBS in the lower chambers served as chemoattractant. The non-invading cells were gently removed with a cotton swab and those migratory cells located on the lower side were stained with crystal violet. The number of migrated cells were counted and averaged in randomly selected 10 fields under microscope. For wound-healing assay, cells were grown into confluent monolayers and scratched using a sterile 200 μl pipette. Cell migration was observed at various time-points later by microscopy. Images of 10 scratches per cells were captured during the experiment and compared with Image J software.
Senescence β-Galactosidase Cell Staining
Senescence β-galactosidase cell staining was performed using staining kit purchased from Cell Signaling (#9860) and performed accordingly. Briefly, cells were fixed in 2% formaldehyde/0.2% glutaraldehyde/PBS for 15 min at room temperature and stained using β-galactosidase staining solution at 37 °C overnight. The percentage of SA-β-gal-positive cells was calculated from 10 randomly chosen fields. At least 200 cells were analyzed per experiment.
Colony-Forming Assay
One thousand shBmi1-transfected and control cells or cells pretreated with TSA, NaB or vehicle were placed into six-well plates or dishes and allowed to grow for 2 weeks. The cells were then fixed and stained with crystal violet. The colonies were further visualized under an invert microscope and photographed. Cell aggregations with >50 cells were defined as colonies and counted.
TSCC Xenograft Model and NaB Treatment
All the animal protocols were in accordance with institutional animal welfare guidelines of Nanjing Medical University. Two million Bmi1 knockdown and control cells (HN4-shBmi1, HN4-NC) were injected subcutaneously on the right flank of male nu/nu mice (8-week old). After tumor growth was monitored for 24 consecutive days, the animals were killed and tumor samples were harvested for further analyses. For drug-treatment animal experiments, 1.5 × 106 viable HN4 cells were inoculated subcutaneously. Four weeks later, these mice bearing tumors were randomly divided into two groups (six mice per group), which were scheduled to receive the following treatments: 150 mg/kg NaB, once every 2 days by intraperitoneal injection or vehicle (PBS) only in controls for consecutive 12 days. The tumor diameters were measured by calipers every 3 days. Tumor volume was calculated by the formula volume (mm3)=(length (mm) × width (mm)2) × 0.5. Tumor weight was also measured upon tumor samples were harvested.
Patients and Tissue Specimens
A total number of 52 patients with primary TSCC treated at the Department of Oral and Maxillofacial Surgery, Nanjing Medical University from 2001 to 2011 were collected. These patients were diagnosed as primary TSCC without any prior history of chemotherapy or radiotherapy and underwent radical tumor ablation and neck lymph node dissection (elective or therapeutic neck dissection as required) alone. The archived tissue samples were retrieved and hematoxylin–eosin staining slides for each patient were further analyzed to confirm the previous histological diagnoses based on the established histological criteria. Sixteen samples of normal tongue mucosa were obtained from non-cancer-related surgeries during the same period and histomorphologically verified. All these patients gave written informed consent in line with our institutional guidelines. This study protocol was reviewed and approved by the Research Ethic Committee of Nanjing Medical University.
Histopathological Evaluation and Immunohistochemistry
Immunohistochemical staining for Bmi1 and Ki-67 was performed and scored similarly as our previous reports.34, 35 The immunoreactivity in each slide was assessed independently by two senior oral pathologists without knowledge about the clinical and pathological information. Negative controls (without primary antibody incubation) were included in each staining run. Immunoreactivity was semiquantitatively evaluated on the basis of staining intensity and distribution using the immunoreactive score, which was calculated as intensity score × proportion score. The immunoreactivity of each slide was divided into three groups based on the final score: 0, negative; 1–4, low expression; 4–12, high expression.
Statistical Analysis
All quantitative data in this study was shown as mean±s.d. of two or three independent experiments and compared with Student's t-test or ANOVA unless otherwise specified. The associations between Bmi1/Ki-67 expression and various clinicopathological parameters were evaluated using Fisher’s exact test or χ2-test as indicated. The overall survival rate was estimated using Kaplan–Meier method and compared with log-rank test. The prognostic analyses were performed by univariate and multivariate Cox regression models to determine the clinicopathological variables with overall survival. P-values <0.05 (two-sided) were considered statistically significant. All statistical analyses were performed using GraphPad Prism 5 or SPSS 18.0 software.
RESULTS
Bmi1 is Aberrantly Overexpressed in TSCC Cell Lines and Clinical Specimens
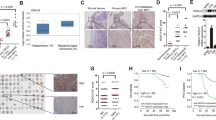
To initially explore the Bmi1 expression in TSCC, we first evaluated both mRNA and protein levels of Bmi1 in a panel of TSCC cell lines as compared with normal tongue mucosa. As shown in Figure 1a, Bmi1 mRNA levels in the cancerous cells except the Tca8113 were significantly higher than that in normal mucosa as assessed by real-time RT-PCR assay. The amounts of Bmi1 mRNA in Cal27, SCC9, SCC25, HN4, HN6, HN12 were elevated approximately 3.45, 3.71, 3.57, 8.42, 5.82 and 6.21-folds (P<0.01), respectively. The following western blotting results further confirmed the significantly upregulated Bmi1 expression in the TSCC cells lines except Tca8113 (Figure 1b). To characterize the subcellular distribution of Bmi1 in tongue cancer cells, cellular immunofluorescence was performed in three selected cell lines. As shown in Figure 1c, Bmi1 was readily detected and mainly identified in nucleus in these cancer cell lines examined. To further examine Bmi1 expression in clinical specimens, we next evaluated the expression patterns of Bmi1 by immunohistochemical staining in a retrospective cohort of 52 primary TSCC samples. In accordance with our immunohistochemistry scoring method, Bmi1 expression patterns in TSCC and normal tongue mucosa were shown in Figure 1e. Bmi1 abundance in these TSCC specimens were graded as low (16) or high expression group (36), whereas its expression levels in normal counterparts were divided into negative (4), low (9) and high (3) group, a clear indicative of aberrant overexpression of Bmi1 in a major fraction of TSCC samples (P<0.01). A representative labeling of Bmi1 in TSCC and normal tongue mucosa was shown in Figure 1d. Consistent with the chromatin-modifying roles of Bmi1 in nucleus, high Bmi1 expression was identified mainly in nucleus but rarely in cytoplasm in cancer cells. Collectively, these data indicate that Bmi1 is aberrantly overexpressed in a significant fraction of tongue cancers.
Overexpression of Bmi1 and its prognostic significance in TSCC. (a) Bmi1 mRNA levels were measured by real-time RT-PCR in seven TSCC cell lines as compared with normal tongue mucosa (n=3). (b) Bmi1 protein levels were determined by western blot (WB) in TSCC lines as compared with normal tongue mucosa. Representative images of WB are shown. (c) Localization of Bmi1 (mainly in nucleus) was identified by immunofluorescence staining in tongue cancer cells. Representative immunofluorescence images are shown. (d) Bmi1 expression in human TSCC specimens was evaluated by immunohistochemical staining ( × 200). Left to right: negative expression in normal tongue mucosa; low expression in TSCC; high expression in TSCC. Upper panel: HE staining; Lower panel: IHC staining. (e) Expression patterns of Bmi1 in human TSCC samples and correlation between Bmi1 and Ki-67 were statistically determined. (f) Overall survival analysis of patients with high or low expression of Bmi1 was estimated by Kaplan–Meier method and compared with log-rank test. Data shown here are mean±s.d. from three independent experiments. **P<0.01, ANOVA analysis.
Overexpression of Bmi1 Associates with Cervical Nodes Metastasis, Ki-67 Expression and Poor Prognosis of TSCC
To dissect the clinical relevance of Bmi1 overexpression in tongue cancers, we next set out to identify potential associations between Bmi1 expression and patients’ clinicopathological parameters. The detailed relationships between Bmi1 status and several clinicopathological variables from 52 patients were shown in Table 1. In brief, 28 male and 24 female patients were enrolled with mean age 53.1 years (26–76 years). The follow-up period ranged from 2 to 102 months with average 43.8 months. There were no significant correlations between Bmi1 expression with patients’ age, gender, tumor size, local invasion, pathological grade and clinical stage. Noticeably, significant association between Bmi1 abundance with cervical nodes metastasis was found with P-values 0.0367. Given the putative pro-proliferative roles of Bmi1 during cancer progression, we determined whether a positive correlation between Bmi1 and Ki-67 existed in TSCC. The expression of canonic cell proliferation marker Ki-67 was evaluated by immunohistochemical staining in the same patient cohort. Indeed, as shown in Figure 1e, Bmi1 expression was found to be significantly associated with Ki-67 (P=0.0384) in these patients analyzed.
To further reveal clinical significance of Bmi1 expression in TSCC patients’ prognosis, we then evaluated the correlation between Bmi1 expression and clinical outcomes. At the time of the last follow-up, 27 of 52 (51.9%) patients were alive and disease-free, 5 (9.6%) patients with locoregional recurrence and/or cervical nodal metastases, 20 (38.5%) patients died because of local recurrence, metastases or other unrelated diseases. In a Kaplan–Meier survival analysis, as shown in Figure 1f, high Bmi1 expression in TSCC was significantly associated with reduced overall survival (log-rank, P=0.0179), suggesting that Bmi1 overexpression associated with adverse prognosis.
To assess whether Bmi1 expression can be identified as a prognostic predictor for TSCC patients, both univariate and multivariate survival analyses (Cox proportional hazards regression model) were performed. In the univariate survival analysis, Bmi1 expression and cervical nodal metastasis significantly associated with overall survival (P=0.002, 0.034, respectively), whereas other clinicopathological variables did not reach the statistical significance as indicated in Table 2. To rule out confounding factors, multivariate survival analysis was carried out with relevant covariates. In this Cox regression model, together with cervical nodal metastasis (P=0.023), Bmi1 expression status was found to be an independent prognostic marker for the overall survival of TSCC patients (P=0.010). Together, these data reveal that Bmi1 is an important diagnostic and prognostic biomarker with incompletely recognized oncogenic functions in tongue cancer.
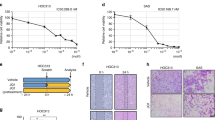
Bmi1 Knockdown Inhibits Cell Proliferation and Migration, and Induces Cell Apoptosis in Tongue Cancer Cell
These above findings from our studies and others have strongly suggested that Bmi1 may be a key oncogenic driver during tongue tumorigenesis.11, 27 However, the detailed pathological roles of Bmi1 underlying this process have not been completely appreciated, which prompted us to further unravel the oncogenic functions of Bmi1 and relevant mechanisms. The shRNA-mediated knockdown strategy was exploited to delineate the resulting phenotype changes after endogenous Bmi1 was specifically inhibited in vitro. As shown in Figures 2a and b, both mRNA and protein of Bmi1 were remarkably reduced following the shBmi1 plasmids transfection into cells as compared with nonspecific control. The shBmi1 plasmid with more knockdown potency (shBmi1-1 for HN4 cells and shBmi1-2 for HN12 cells, respectively) was identified and utilized in the following experiments. In order to further confirm the Bmi1 knockdown in vitro, the expression changes of two downstream targets of Bmi1, p16 and p14, which were normally silenced by Bmi1 were determined following Bmi1 knockdown. Our data from real-time RT-PCR assay indicated that they were derepressed and significantly upregulated in HN4 and HN12 cells when endogenous Bmi1 was depleted (Supplementary Figure 1), thus displaying the potency and specificity of the shBmi1-mediated knockdown. Subsequently, the relevant phenotype changes of cells after Bmi1 silencing were further examined in detail. As shown in Figure 2c, Bmi1 depletion resulted in impaired cell proliferation in both cells as evidenced by the facts that shBmi1-transfected HN4 and HN12 displayed much lower cell viability at 72 and 96 h after transfection as compared with control, although the inhibitory effects were not much obvious at 24 and 48 h. In addition, the Annexin V and PI double staining data indicated that 48 h after transfection, the percentages of cells undergoing apoptosis were higher in shBmi1-treated cells when compared with controls (HN4 4.96% vs 9.68%; HN12 5.32% vs 11.25%; P<0.01; Figure 2d), suggesting that Bmi1 silencing induced cell apoptosis in tongue cancer cells. Moreover, shBmi1-transfected cells displayed impaired migration capacity as shown in Figure 2e. In line with this, gain of E-cadherin and loss of Vimentin follow Bmi1 knockdown were detected in both cell lines, which presumably accounted for the impaired immigration (Figure 2f). Collectively, these results provide evidence that Bmi1 is implicated in cell proliferation, apoptosis and migration in tongue cancer cell.
Bmi1 knockdown inhibits cell proliferation and migration, and triggers cell apoptosis in tongue cancer cells. (a) Endogenous Bmi1 was efficiently silenced by shBmi1 in two tongue cancer cell lines. Representative images of WB are shown. (b) The mRNA levels of Bmi1 were significantly decreased following Bmi1 knockdown. (c) Cell proliferation was remarkably suppressed when endogenous Bmi1 was silenced as measured by MTT assay. (d) Increased percentages of cell undergoing apoptosis were evident in shBmi1-transfected cells as assayed by Annexin V-PI double staining. (e) The migration ability was significantly reduced in shBmi1-treated cells in relative to control. The numbers of migrated cells were calculated in randomly selected 10 fields under microscope and then averaged (lower panel). (f) The abundances of E-cadherin and Vimentin were measured by WB following endogenous Bmi1 knockdown. Representative images are shown. Data shown here are mean±s.d. from three independent experiments, *P<0.05, **P<0.01, Student’s t-test.
Bmi1 Knockdown Reduces Cell Cloning Potential, Percentage of CD44+CD133+ Cell Sub-Population, Triggers Cell Senescence and Enhances Cisplatin Chemosensitivity
Given the essential roles of Bmi1 in cancer initiation and progression, in particular cancer stem cell maintenance and function, senescence and chemoresistance,17, 36 we then asked whether Bmi1 knockdown affected these properties in tongue cancer cells. First, the colony-forming assay was performed to evaluate the roles of Bmi1 in the colony-forming efficiency, which is a surrogate readout of stem cell abundance. The results indicated that the numbers of colony were much fewer in shBmi1-treated cells than those in control cells (P<0.05; Figure 3a). In addition, the sizes of colonies were also markedly smaller in shBmi1-treated cells as compared with control. Then, we next sought to measure Bmi1 in the CD44 and CD133 double-positive cell sub-population, which enriched putative cancer stem cells by fluorescent-activated cell sorting.37, 38 Following cell sorting, these cell fractions with both CD44 and CD133 positive and negative were subjected to western blot assay. Significantly elevated Bmi1 was observed in CD44+CD133+ fraction as compared with CD44−CD133− fraction and unsorted parental population (Supplementary Figure 2). Notably, the percentage of CD44+CD133+ cell fraction decreased significantly from 20.8 to 15.9% in HN4 and 10.2 to 3.17% in HN12 following Bmi1 knockdown as shown in Figure 3b. These two cell surface markers CD44 and CD133, commonly used for cancer stem cell identification and enrichment in multiple cancers, were significantly downregulated in cells with Bmi1 silencing (Supplementary Figure 3). These data suggested important roles of Bmi1 in maintaining CD44+CD133+ sub-population in tongue cancer. As shown in Figure 3c, increased SA-β-gal staining positive cells were observed in cells transfected with shBmi1 as relative to control (P<0.01), indicating Bmi1 knockdown triggered cells to undergo senescence. As the key roles of Bmi1-mediated p16 expression during cell senescence,39 p16 mRNA and protein were both significantly elevated after Bmi1 silencing, further confirming the Bmi1-p16 involvement in regulation of tongue cancer cell senescence (Figure 3d and Supplementary Figure 1). Previous reports have offered clues that aberrantly overexpression of Bmi1 associated with chemoresistance and its depletion enhanced chemosensitivity in multiple cancers.36 Then, we wondered whether Bmi1 knockdown can enhance antitumor activities with the commonly used anticancer drug cisplatin. Not surprisingly, cell viability was significantly impaired when cells were treated with cisplatin or shBmi1 alone as measured by MTT assay. Remarkably, cell viability and proliferation were more significantly reduced in shBmi1 cells treated with cisplatin in comparison with those cells with cisplatin (Figure 3e). Furthermore, the percentage of apoptotic cells was much higher in shBmi1 cells with cisplatin incubation than that in cells with cisplatin only (data not shown). Together, these findings suggest that Bmi1 is involved in cancer stem cell maintenance, cell senescence and chemotherapeutic sensitivity in tongue cancer cell.
Bmi1 knockdown reduces colony formation, CD44+CD133+ sub-population, triggers cell senescence, and enhance cisplatin chemosensitivity in tongue cancer cells. (a) The colony formation efficiency was pronouncedly reduced in shBmi1 cells as compared with control. (b) The percentages of CD44+CD133+ sub-population enriched with putative cancer stem cells were decreased after Bmi1 knockdown as measured by FACS. (c) The proportion of cell undergoing senescence was markedly increased following Bmi1 silencing as determined by SA-β-gal staining. (d) The abundance of p16 as cell senescence regulator was measured by WB assay. Representative images of WB are shown. The same GAPDH blot was used in Figure 3d and Figure 2f from a representative experiment. (e) Cell proliferation was significantly inhibited in shBmi1 cells treated by cisplatin (2.5 μg/ml) as assayed by MTT. Data shown here are mean±s.d. from three independent experiments, *P<0.05, **P<0.01, Student’s t-test and ANOVA analyses.
HDACi Inhibits Bmi1 Expression in Tongue Cancer Cells
Several lines of evidence have indicated that the members of PRC such as Bmi1 are identified as transcriptional targets of HDACi and several HDACis have capacities to repress Bmi1 and derepressed its downstream targets in various cancer cells.23, 24, 25 Then we sought to confirm whether HDACis were capable to repress Bmi1 expression in tongue cancer cells. Two HDACis, TSA and NaB, were used to evaluate their effects on Bmi1 abundance. HN4 cells were incubated with various concentrations of TSA (0–400 nM) and NaB (0–4 μM) for varying time courses. As shown in Figures 4a and b, TSA and NaB exposure induced a time-dependent and dose-dependent decrease of Bmi1 protein in HN4 cells. In addition, another key member of polycomb complex EZH2 was also inhibited by both TSA and NaB in vitro (Figure 4c). Moreover, TSA and NaB treatment significantly inhibited mRNA levels of Bmi1 and EZH2, and derepressed p16 simultaneously (Figure 4d). Taken together, these data indicate that TSA and NaB have capacities to inhibit Bmi1 expression in tongue cancer cell.
Bmi1 is inhibited by two HDACi in HN4 tongue cancer cells. (a, b) TSA and NaB inhibited Bmi1 in a dose- and time-dependent manner in HN4 cells. Representative images of WB are shown. (c) EZH2 was also inhibited by TSA (200 nM) and NaB (2 μM) in HN4 cells. Representative images of WB are shown. (d) Bmi1, EZH2 and p16 mRNAs were measured by real-time RT-PCR assay after cells were treated with TSA (200 nM) and NaB (2 μM) for 48 h. Data shown here are mean±s.d. from three independent experiments, *P<0.05, **P<0.01, ANOVA analyses.
HDACi-Induced Bmi1 Depletion Phenocopies shRNA-Mediated Bmi1 Silencing In Vitro
We next want to clarify the effects of TSA and NaB on cell phenotypic changes in tongue cancer cells and whether these changes resemble those of Bmi1 knockdown. In general, the resultant effects induced by TSA and NaB exposure largely phenocopied the changes following Bmi1 knockdown in HN4 cells. As displayed in Figures 5a–f and Supplementary Figure 4, our results indicate that both TSA and NaB treatment result in impaired cell proliferation, reduced migration and cell colony formation, more apoptotic and senescence cells, reduced proportion of CD44+CD133+ cell fractions and enhanced cisplatin sensitivity, largely resembling the results derived from Bmi1 silencing in vitro. However, unexpectedly, significant morphological changes were observed in TSA-treated cells, whereas no such morphological changes were detected in NaB-treated cells. As shown in Supplementary Figures 5A and B, TSA-treated cells displayed EMT-like characteristics such as spindle fibroblast-like shape and loss of cell–cell adhesion, whereas NaB-treated cells remained unchanged with typical epithelial morphology. In addition, EMT marker E-cadherin was significantly decreased and Vimentin was remarkably upregulated in TSA-treated HN4 cells, whereas NaB appeared to induce the opposite changes as E-cadherin gain and Vimentin loss (Supplementary Figures 5B and C). This finding suggested that TSA may have the potential to trigger EMT in tongue cancer cells. To discriminate whether such morphological changes were due to specific cell type or specific HDACi chemicals, we then tested both TSA and NaB in another two cell lines HN6 and Cal27. The data indicated that only TSA treatment can result in EMT-like morphological changes and E-cadherin loss in HN6 cells (Supplementary Figures 5A, C and D) and Cal27 cells (data not shown). As expected, TSA exposure significantly enhanced cell migration, whereas NaB treatment remarkably impaired cell migration in both HN4 and HN6 cells (Supplementary Figure 5E and data not shown). Taken together, these data reveal that HDACis TSA and NaB have potent anticancer effects in tongue cancer cells. TSA, not NaB, had unexpected potential to induce EMT-like changes in tongue cancer cells.
The effects of HDACi treatments largely phenocopy Bmi1 knockdown in HN4 tongue cancer cells. (a) Cell proliferation was significantly impaired in HN4 cells after TSA and NaB treatments as measured by MTT assay. (b) The percentages of apoptotic cells were remarkably increased in HN4 cells after TSA and NaB treatments as determined by flow cytometry and cleaved-PARP expression (WB). (c) SA-β-gal staining positive cells were determined and compared after HN4 cells were treated with TSA and NaB for 72 h. (d) The percentages of CD44+CD133+ sub-population were decreased significantly in TSA or NaB-treated HN4 cells as determined by FACS. (e) The colony formation efficiencies were impaired in HN4 cells in the presence of TSA or NaB. (f) Cell viability and proliferation was determined in HN4 cells treated with TSA/NaB alone or in combination with cisplatin. The dosages of TSA and NaB used here were 200 nM and 2 μM unless other specified. Data shown here are mean±s.d. from three independent experiments, *P<0.05, **P<0.01, Student’s t-test and ANOVA analyses.
NaB Induces Anticancer Effects on Tongue Cancer Cells in Part by Bmi1 Downregulation
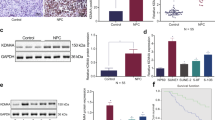
Given that HDACi has multiple targets depending on cellular and biological contexts, these observed effects induced by HDACi treatment in tongue cancer cells may be directly or indirectly or even not associated with Bmi1 repression. To address this, we performed the rescue experiments in vitro by enforced overexpression of Bmi1 following NaB pretreatment. The Bmi1 expression vector (pcDNA3-Bmi1) was first verified by transiently delivery into Cal27 cells, which have relative low endogenous Bmi1. As shown in Supplementary Figure 6, the abundance of Bmi1 was markedly increased relative to control (pcDNA3-empty). Consistently, the expected changes of p16, E-cadherin and Vimentin proteins were observed simultaneously, thus confirming the efficiency of this Bmi1 expression construct. After pretreated with NaB (2 μM) for 48 h, then these cells received transient transfection with pcDNA3-Bmi1 or pcDNA3-empty. Following another 48 h after transfection, these cells were harvested for analyses. As shown in Figure 6a, the pcDNA3-Bmi1 transfection was capable to partially rescue the Bmi1 expression after NaB pretreatment, whereas the empty plasmid failed. More importantly, the effects on cell proliferation, apoptosis and migration induced by NaB were partially relieved by enforced Bmi1 overexpression (Figures 6b, c and d). Collectively, our data suggest that these anticancer effects triggered by NaB on tongue cancer cells were attributed at least in part to Bmi1 downregulation.
NaB induces therapeutic effects in part via Bmi1 inhibition in tongue cancer cells. (a) Enforced Bmi1 overexpression attenuated Bmi1 inhibition induced by NaB pretreatment. These cell underwent NaB exposure for 48 h and then were transfected with Bmi1 vectors (pcDNA3-Bmi1or empty as control) for another 48 h. The abundance of Bmi1 and its downstream target p16 were measured by WB. Representative images are shown. (b–d) Following NaB pretreatment for 48 h and Bmi1 plasmids (pcDNA3-Bmi1 or empty as control) transfection for 48 h, these cells were harvested for cell proliferation (b), apoptosis (c) and migration assay (d). Data shown here are mean±s.d. from two independent experiments, NS, nonsignificant, *P<0.05, **P<0.01, Student’s t-test and ANOVA analyses.
Genetic and Pharmacologic Inhibition of Bmi1 Inhibited Tumor Growth In Vivo
To further reinforce the notion that Bmi1 is critical for tongue cancer growth and testify the therapeutic efficiency of NaB in vivo, both genetic and pharmacologic approaches were further utilized to inhibit Bmi1 in a tongue cancer xenograft model. Equal amount of HN4 Bmi1-knockdown cells (HN4-shBmi1) and control cells (HN4-NC) were transplanted subcutaneously into flanks of nude mice. As shown in Figures 7a and b, the data indicated that the tumor grew much faster in control cells transplantation as compared with Bmi1-knockdown cells transplantation as measured by tumor volume and final tumor weight. Then, we went on to testify the in vivo effects of NaB in tongue cancer growth. After subcutaneous inoculation of HN4 cells for 4 weeks, tumor masses were established and then mice were randomly grouped for drug treatment (NaB or vehicle by intraperitoneal injection). The data derived from tumor volume and weight clearly indicated that NaB treatment significantly retarded tumor growth, although tumor regression was not achieved (Figures 7c and d). The immunohistochemical staining data displayed remarkably inhibited Bmi1 expression in NaB-treated samples as compared with vehicle-treated samples (Figure 7e). Together, these findings reveal that Bmi1 is required for tongue cancer growth in vivo and NaB treatment impaired tongue cancer growth likely via Bmi1 inhibition.
Genetic silencing of Bmi1 or pharmacological inhibition of Bmi1 by NaB impairs tumor growth in a TSCC xenograft model. (a) Volume changes in tumor masses formed from HN4-shBmi1 and HN4-NC cells. (b) Final tumor weight in samples harvested from mice bearing HN4-shBmi1 and HN4-NC cells. (c) Volume changes from two animal groups treated with NaB (150 mg/kg/day) or vehicle (PBS) after tumor masses were established 4 weeks after subcutaneous inoculation. (d) Final tumor weight in samples harvested from mice treated with NaB (150 mg/kg/day) or vehicle (PBS). (e) HE (magnification: × 200) and immunohistochemical staining (IHC, magnification: × 200 and 400) in two tumor samples from mice treated with NaB (150 mg/kg/day) or vehicle (PBS), respectively. Representative images are shown. Images in lower panel (magnification: × 400) showed the magnification of black-labeled parts in middle panel (magnification: × 200). *P<0.05, **P<0.01, Student’s t-test.
DISCUSSION
The polycomb group proteins maintain gene expression by modifying chromatin structure and involve in various fundamental biological processes including stem cell plasticity, cell differentiation and proliferation.5, 40 Mounting evidence has demonstrated that Bmi1, the core component of PRC1, has been increasingly recognized as a key bona fide oncogene underlying tumorigenesis and a potential therapeutic target against human cancer.19 In this study, our data reveal that Bmi1 is abnormally overexpressed in a large fraction of tongue cancers and its overexpression associates with aggressive malignant characteristics and patients outcomes. Moreover, using genetic knockdown and pharmacological approach, multiple oncogenic roles of Bmi1 were identified in cancer cell proliferation, cellular apoptosis and senescence, and CD44+CD133+ sub-population maintenance during tongue cancer progression. HDACs NaB was capable to induce therapeutic effects on tongue cancer cells presumably through Bmi1 inhibition both in vitro and in a xenograft animal model.
Accumulating evidence has indicated that Bmi1 represents an oncogene that was strongly linked to cell transformation, EMT and cancer stem cell propagation in diverse cancer contexts including oral cancers.8, 12, 41 Previous studies have reported that ectopic Bmi1 overexpression results in senescence bypass and immortalization of normal keratinocytes and associates with malignant progression of precancerous oral lesion into cancers.41, 42 Moreover, overexpression of Bmi1 occurred at a very early stage in oral carcinogenesis and was required for cancer cell proliferation and survival.30 Our data further revealed aberrant overexpression patterns of Bmi1 in tongue cancers. Bmi1 upregulation associated with clinical aggressive features and patient prognosis. However, our finding is inconsistent with a previous study, which revealed that Bmi1 expression did not correlate with any clinical and histopathological parameters and its negative expression may serve as a poor prognostic marker in patients with primary T1N0M0 tongue cancers.26 We reasoned that such discrepancy may be partially due to different patient’s inclusion criteria, sample volume and immunohistochemical scoring protocol. Therefore, our results together with most previous studies indicate that aberrant Bmi1 overexpression may be one of the pivotal molecular events during tongue tumorigenesis and also a novel biomarker for tongue cancer with diagnostic and prognostic significance.
The clinicopathological significance of Bmi1 overexpression in tongue cancers prompted us to further dissect the biological roles of Bmi1 during cancer progression by loss-of-function assay using genetic knockdown in vitro and in vivo. Our data reveal that Bmi1 regulates cell proliferation, cell apoptosis and senescence, and cell motility in tongue cancer cells, which generally agree with the previously reported oncogenic functions of Bmi1 in diverse human cancers.5, 19 A line of evidence has established that Bmi1 has multiple tumorigenic roles in cancer progression by regulating a myriad of target genes. We believe that these well-established downstream targets such as p16, p14 and E-cadherin are largely responsible for Bmi1-mediated phenotypes in tongue cancer. For example, derepressed p16 and p14 following endogenous Bmi1 silencing presumably accounted for impaired cell proliferation and cellular senescence induction, because the well-known functions of p16 and p14 involved in cell cycle progression and premature senescence have long appreciated.18, 19, 43 The close correlation between Bmi1 and Ki-67 expression in clinical samples also supported the pro-proliferative roles of Bmi1 conferred on tongue cancer cells. The in vivo data from xenograft animal model provided further evidence that Bmi1 is required and critical for tongue cancer growth.
Bmi1 has critical roles implicated in normal and malignant stem cell self-renewal and maintenance in several tissues.7, 13 In particular, Bmi1 was recently identified as a key marker of lingual epithelial stem cells, which were important for tissue maintenance and regeneration of lingual epithelium.44 This unique cell sub-population with tumor-initiating properties, termed cancer stem cells, is phenotypically identified in head neck cancers with cell surface markers CD44, CD133 or ALDH1 and thought to be critically responsible for cancer initiation, progression and therapeutic resistance.45 For example, Bmi1 was highly enriched in CD133+ laryngeal cancer stem cells and critically required for their proliferation and expansion.46 Properties of cancer stem cell in human colorectal cancer are highly dependent on Bmi1, because downregulation of Bmi1 inhibits their self-renewal and abrogates their tumorigenic potential in vitro and in animal models.20 Not surprisingly, consistent with these findings, Bmi1 was found significantly enriched in CD44+CD133+ sub-population containing cancer stem cell from tongue cancer lines. Bmi1 depletion inhibited colony formation, reduced the percentage of cancer stem cell population with accompanying downregulation of CD44 and CD133. Moreover, these Bmi1-depleted cell appeared to be more vulnerable to conventional anticancer agent cisplatin and displayed significantly impaired proliferation. These data strongly suggest that Bmi1 may be indispensable for cancer stem cell maintenance and implicated in cisplatin-related chemoresistance in tongue cancer. However, further in-depth investigations into the molecular roles of Bmi1 in tongue cancer stem cell homeostasis are warranted.
Recently, it has been reported that the HDACi inhibited the core members of polycomb group proteins, Bmi1 and EZH2 in particular, in multiple cancer contexts.23, 25 Specifically, HDACi can downregulate the expression of Bmi1 by transcriptional mechanism in breast cancer cells.23 Bmi1 was also significantly repressed by diverse HDACi chemical inhibitors during mesenchymal stem cell senescence and phenotypic alternation of cancer stem cells derived from head neck cancers.24, 47 Thus, these findings suggested that Bmi1 can be robustly inhibited by HDACi in different biological contexts and offered some valuable clues that HDACi chemical compounds may have anticancer properties by suppressing Bmi1 in addition to other known targets and molecular pathways. In agreement with these, our results clearly indicate that Bmi1 is potently inhibited by two different HDACi in tongue cancer cells in vitro. Furthermore, this inhibition may be attributed to transcriptional repression induced by HDACi, because we reasoned that increased acetylation of core histone resulting from HDACi impaired transcription factor accessibility and function, thereby favoring gene silencing.48 The decreased mRNAs of Bmi1 upon HDACi treatment also supported this notion. Of course, here we cannot rule out the possibility that Bmi1 inhibition may be indirectly induced by HDACi besides transcriptional silencing and other mediators exist for HDACi anticancer functions, which is needed to further clarified and confirmed. Generally, similar with the effects induced by HDACi in other cancers, these chemical compounds impaired cell growth as well as induced cell apoptosis and senescence in tongue cancer.49, 50 Moreover, HDACi exposure also significantly reduced cell colony formation and CD44+CD133+ cell fraction, and enhanced cisplatin sensitivity in tongue cancer cells. These phenotypic alternations induced by HDACi largely phenocopied the Bmi1 knockdown. Moreover, our in vitro rescue experiments indicate that ectopic Bmi1 overexpression was capable to attenuate the repression of Bmi1 induced by NaB exposure and partially attenuate the resulting phenotypic changes after NaB treatment. These findings support the idea that the therapeutic effects of HDACi NaB were as least in part due to its inhibition of Bmi1. Cancer stem cell sub-population in tongue cancer may be efficiently targeted by HDACi, which is quite beneficial to reduce disease recurrence and therapeutic resistance. Our findings from the xenograft animal model showed that NaB treatment alone significantly inhibited tumor growth, thus offering valuable clues to further determine the therapeutic efficiency of HDACi alone or in combination with conventional agents to eradicate tongue cancers.
Previous studies have indicated that HDACi treatment have the capacities to both inhibit and induce EMT program in diverse cell types and biological contexts.51, 52, 53, 54 On the one hand, HDACi chemicals reverted the mesenchymal phenotype by inducing E-cadherin gain and vimentin loss in gefitinib-resistant KB and Hep-2 cell undergoing EMT and murine hepatocytes with TGF-β1-induced EMT.53, 54 However, on the other hand, some HDACi unexpectedly triggered EMT program via Snail upregulation in human nasopharyngeal carcinoma cell lines CNE2 and HepG2, and head neck cancer cell lines HN6 and HN13.52, 55 In our study, TSA appeared to paradoxically induce EMT-like changes such as morphological alternation, E-cadherin gain and vimentin loss and enhanced cell motility in tongue cancer cell lines, whereas NaB had no such unexpected capabilities. These findings raise the possibility that some other uncharacterized targets of HDACi independent of Bmi1 may be responsible for these contradictory EMT changes. It will be interesting to further comprehensively dissect the anticancer functions of diverse HDACi, which is an essential prerequisite before these chemicals were translated into the clinic.
In this study, our findings indicate that Bmi1 serves as a key driver with multiple oncogenic functions during tongue cancer progression and as a novel biomarker for diagnosis and prognostic prediction for patients. HDACi chemicals such as NaB induce therapeutic effects against tongue cancer probably in part by Bmi1 repression. We anticipate that pharmacological and genetic disruption of Bmi1 may represent a novel therapeutic strategy against tongue cancers.
References
Haddad RI, Shin DM . Recent advances in head and neck cancer. N Engl J Med 2008;359:1143–1154.
Ferlay J, Shin HR, Bray F et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–2917.
Siegel R, DeSantis C, Virgo K et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–241.
Scully C, Bagan JV . Recent advances in oral oncology 2008; squamous cell carcinoma imaging, treatment, prognostication and treatment outcomes. Oral Oncol 2009;45:e25–e30.
Sauvageau M, Sauvageau G . Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 2010;7:299–313.
Jacobs JJ, Kieboom K, Marino S et al. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999;397:164–168.
Lukacs RU, Memarzadeh S, Wu H et al. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 2010;7:682–693.
Yang MH, Hsu DS, Wang HW et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010;12:982–992.
Song LB, Zeng MS, Liao WT et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 2006;66:6225–6232.
Mohty M, Yong AS, Szydlo RM et al. The polycomb group BMI1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood 2007;110:380–383.
Guo BH, Feng Y, Zhang R et al. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer 2011;10:10.
Dovey JS, Zacharek SJ, Kim CF et al. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA 2008;105:11857–11862.
Gargiulo G, Cesaroni M, Serresi M et al. In vivo RNAi screen for BMI1 targets identifies TGF-beta/BMP-ER stress pathways as key regulators of neural- and malignant glioma-stem cell homeostasis. Cancer Cell 2013;23:660–676.
Meng X, Wang Y, Zheng X et al. shRNA-mediated knockdown of Bmi-1 inhibit lung adenocarcinoma cell migration and metastasis. Lung Cancer 2012;77:24–30.
Wu Z, Min L, Chen D et al. Overexpression of BMI-1 promotes cell growth and resistance to cisplatin treatment in osteosarcoma. PLoS One 2011;6:e14648.
Smith LL, Yeung J, Zeisig BB et al. Functional crosstalk between Bmi1 and MLL/Hoxa9 axis in establishment of normal hematopoietic and leukemic stem cells. Cell Stem Cell 2011;8:649–662.
Chiba T, Miyagi S, Saraya A et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res 2008;68:7742–7749.
Bennecke M, Kriegl L, Bajbouj M et al. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 2010;18:135–146.
Cao L, Bombard J, Cintron K et al. BMI1 as a novel target for drug discovery in cancer. J Cell Biochem 2011;112:2729–2741.
Kreso A, van Galen P, Pedley NM et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014;20:29–36.
West AC, Johnstone RW . New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014;124:30–39.
Iglesias-Linares A, Yanez-Vico RM, Gonzalez-Moles MA . Potential role of HDAC inhibitors in cancer therapy: insights into oral squamous cell carcinoma. Oral Oncol 2010;46:323–329.
Bommi PV, Dimri M, Sahasrabuddhe AA et al. The polycomb group protein BMI1 is a transcriptional target of HDAC inhibitors. Cell Cycle 2010;9:2663–2673.
Jung JW, Lee S, Seo MS et al. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci 2010;67:1165–1176.
Romanski A, Schwarz K, Keller M et al. Deacetylase inhibitors modulate proliferation and self-renewal properties of leukemic stem and progenitor cells. Cell Cycle 2012;11:3219–3226.
Hayry V, Makinen LK, Atula T et al. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br J Cancer 2010;102:892–897.
Chen H, Zhou L, Wan G et al. BMI1 promotes the progression of laryngeal squamous cell carcinoma. Oral Oncol 2011;47:472–481.
Chou CH, Yang NK, Liu TY et al. Chromosome instability modulated by BMI1-AURKA signaling drives progression in head and neck cancer. Cancer Res 2013;73:953–966.
Song LB, Li J, Liao WT et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 2009;119:3626–3636.
Kang MK, Kim RH, Kim SJ et al. Elevated Bmi-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer 2007;96:126–133.
Wang C, Liu X, Chen Z et al. Polycomb group protein EZH2-mediated E-cadherin repression promotes metastasis of oral tongue squamous cell carcinoma. Mol Carcinog 2013;52:229–236.
Douglas D, Hsu JH, Hung L et al. BMI-1 promotes ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res 2008;68:6507–6515.
Li Z, Wang Y, Qiu J et al. The polycomb group protein EZH2 is a novel therapeutic target in tongue cancer. Oncotarget 2013;4:2532–2549.
Wei Z, Wang Y, Li Z et al. Overexpression of Hippo pathway effector TAZ in tongue squamous cell carcinoma: correlation with clinicopathological features and patients' prognosis. J Oral Pathol Med 2013;42:747–754.
Liu LK, Jiang XY, Zhou XX et al. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol 2010;23:213–224.
Siddique HR, Saleem M . Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells 2012;30:372–378.
Prince ME, Sivanandan R, Kaczorowski A et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 2007;104:973–978.
Sinha N, Mukhopadhyay S, Das DN et al. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral Oncol 2013;49:854–862.
Park IK, Morrison SJ, Clarke MF . Bmi1, stem cells, and senescence regulation. J Clin Invest 2004;113:175–179.
Simon JA, Kingston RE . Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 2009;10:697–708.
Liu W, Feng JQ, Shen XM et al. Two stem cell markers, ATP-binding cassette, G2 subfamily (ABCG2) and BMI-1, predict the transformation of oral leukoplakia to cancer: a long-term follow-up study. Cancer 2012;118:1693–1700.
Qiao B, Chen Z, Hu F et al. BMI-1 activation is crucial in hTERT-induced epithelial-mesenchymal transition of oral epithelial cells. Exp Mol Pathol 2013;95:57–61.
Rayess H, Wang MB, Srivatsan ES . Cellular senescence and tumor suppressor gene p16. Int J Cancer 2012;130:1715–1725.
Tanaka T, Komai Y, Tokuyama Y et al. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat Cell Biol 2013;15:511–518.
Gonzalez-Moles MA, Scully C, Ruiz-Avila I et al. The cancer stem cell hypothesis applied to oral carcinoma. Oral Oncol 2013;49:738–746.
Chen H, Zhou L, Dou T et al. BMI1'S maintenance of the proliferative capacity of laryngeal cancer stem cells. Head Neck 2011;33:1115–1125.
Chikamatsu K, Ishii H, Murata T et al. Alteration of cancer stem cell-like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Sci 2013;104:1468–1475.
Chi P, Allis CD, Wang GG . Covalent histone modifications—miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer 2010;10:457–469.
Nagumo T, Takaoka S, Yoshiba S et al. Antitumor activity of suberoylanilide hydroxamic acid against human oral squamous cell carcinoma cell lines in vitro and in vivo. Oral Oncol 2009;45:766–770.
Prystowsky MB, Adomako A, Smith RV et al. The histone deacetylase inhibitor LBH589 inhibits expression of mitotic genes causing G2/M arrest and cell death in head and neck squamous cell carcinoma cell lines. J Pathol 2009;218:467–477.
Kong D, Ahmad A, Bao B et al. Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells. PLoS One 2012;7:e45045.
Giudice FS, Pinto DS Jr., Nor JE et al. Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer. PLoS One 2013;8:e58672.
Bruzzese F, Leone A, Rocco M et al. HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol 2011;226:2378–2390.
Yoshikawa M, Hishikawa K, Marumo T et al. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol 2007;18:58–65.
Jiang GM, Wang HS, Zhang F et al. Histone deacetylase inhibitor induction of epithelial-mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim Biophys Acta 2013;1833:663–671.
Acknowledgements
We thank Dr Liang-Nian Song (Herbert Irving Comprehensive Cancer Center, Columbia University) for critical review and helpful discussion of the manuscript. We are grateful to Professor Wantao Chen for providing cell lines and Professor Muh-Hwa Yang for kind gift of Bmi1 plasmids. This work was supported, in whole or in part, by National Natural Science Foundation of China (Grant no. 81100737), Natural Science Foundation of Jiangsu Province (Grant no. BK2011762, BK20130898), Specialized Research Fund for the Doctoral Program of Higher Education (Grant no. 20113234120003), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Grant no. 2014-37, JX10531802), and China Postdoctoral Science Foundation (2014M560436).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
In this paper, the authors show that B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) is aberrantly overexpressed in a significant fraction of tongue cancers and is associated with cervical node metastasis and reduced overall survival. Functional assays reveal that Bmi1 plays a key role in tongue tumorigenesis, and pharmacologic inhibition of Bmi1 by sodium butyrate significantly inhibits tumor growth in vivo.
Rights and permissions
About this article
Cite this article
Li, Z., Wang, Y., Yuan, C. et al. Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab Invest 94, 1431–1445 (2014). https://doi.org/10.1038/labinvest.2014.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2014.123
This article is cited by
-
Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC
Strahlentherapie und Onkologie (2021)
-
The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma
Cell Death & Disease (2019)
-
TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma
Cancer Cell International (2018)
-
Expression of Cancer Stem Cell Biomarkers in Human Head and Neck Carcinomas: a Systematic Review
Stem Cell Reviews and Reports (2018)
-
Overexpression of suppressor of zest 12 is associated with cervical node metastasis and unfavorable prognosis in tongue squamous cell carcinoma
Cancer Cell International (2017)