Abstract
The mechanisms underlying the involvement of advanced glycation endproducts (AGEs) in diabetic atherosclerosis are not fully understood. The present study was designed to investigate whether intermediate-conductance Ca2+-activated K+ channels (KCa3.1 channels) are involved in migration and proliferation induced by AGEs in cultured rat vascular smooth muscle cells (VSMCs) using approaches of whole-cell patch voltage-clamp, cell proliferation and migration assay, and western blot analysis. It was found that the current density and protein level of KCa3.1 channels were enhanced in cells incubated with AGE-BSA (bovine serum albumin), and the effects were reversed by co-incubation of AGE-BSA with anti-RAGE (anti-receptors of AGEs) antibody. The ERK1/2 inhibitors PD98059 and U0126, the P38-MAPK inhibitors SB203580 and SB202190, or the PI3K inhibitors LY294002 and wortmannin countered the KCa3.1 channel expression by AGE-BSA. In addition, AGE-BAS increased cell migration and proliferation, and the effects were fully reversed with anti-RAGE antibody, the KCa3.1 channel blocker TRAM-34, or KCa3.1 small interfering RNA. These results demonstrate for the first time that AGEs-induced increase of migration and proliferation is related to the upregulation of KCa3.1 channels in rat VMSCs, and the intracellular signals ERK1/2, P38-MAPK and PI3K are involved in the regulation of KCa3.1 channel expression.
Similar content being viewed by others
Main
It is generally recognized that advanced glycation endproducts (AGEs) are formed by the nonenzymatic glycation reaction of aldose sugars with amino groups of proteins.1, 2 In diabetes mellitus, the high-level glucose reacts with protein and forms the adduct AGEs.2, 3 Atherosclerosis and related microvascular disorders are major vascular complications and constitute the increasing morbidity and mortality in diabetes mellitus. Recent studies have demonstrated that AGEs and the receptors of AGEs (RAGE) are upregulated in atherosclerotic plaques in diabetic subjects, particularly in intimal macrophages and smooth muscle cells.4, 5, 6, 7 Accumulation of AGEs and activation of RAGE are found to mediate proliferation, migration, and inflammatory gene expression in vascular smooth muscle cells (VSMCs), which are believed to accelerate the formation of atherosclerosis in diabetes;8, 9, 10 however, the detailed mechanisms underlying atherosclerosis are not fully understood.
Intermediate-conductance Ca2+-activated K+ (KCa3.1 or IKCa) channels are upregulated in the proliferative VSMCs.11, 12, 13, 14 Inhibition of KCa3.1 channels by the selective blocker TRAM-34 is found to inhibit proliferation mediated by platelet-derived growth factor in VSMCs and decrease the development of restenosis.15 Local delivery of TRAM-34 via balloon catheter prevents ion channel phenotype switching (KCa1.1 to KCa3.1) in VSMCs of coronary artery and reduces subsequent restenosis.14 Moreover, Toyama et al16 have provided the additional evidence that upregulation of KCa3.1 channels is related to atherogenesis in mice and humans.
We have recently demonstrated that glycosylated hemoglobin (ie, AGE) level is remarkably increased in a diabetic rat model established by feeding high fat and glucose diet and then followed by injecting a small dose streptozotocin,17 and KCa3.1 channel expression was significantly increased in intimal and medial layers of the aorta in this diabetic rat model.18 In the present study, we investigated whether the effect of AGEs would be related to the activity of KCa3.1 channels in cultured rat VSMCs using electrophysiology and molecular biological approaches.
MATERIALS AND METHODS
Experimental Regents
The ERK1/2 inhibitor PD98059 and U0126, the P38-MAPK inhibitor SB203580, and the PI3K inhibitor LY294002 were purchased from Promega (Madison, WI, USA). Wortamannin and KCa3.1 antibody were obtained from Alomone Laboratory (Jerusalem, Israel). Anti-RAGE antibody was from R&D system (Minneapolis, MN, USA). Anti-bromodeoxyuridine (BrdU) antibody, SB202190, 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) and other chemicals used in this study were obtained from Sigma-Aldrich (St Louis, MO, USA).
Preparation of AGE-BSA
AGE-bovine serum albumin (AGE-BSA) was prepared as described previously.19, 20 Briefly, cell culture-tested BSA with low endotoxin (2.0 g, Sigma-Aldrich) was dissolved in 10 ml of 0.5 mM phosphate-buffered saline (pH 7.4) and 1.6 mM D-glucose, sterilized by ultrafiltration, and then incubated at 37 °C for 12 weeks, followed by dialysis against phosphate-buffered saline for 24 h to remove unincorporated glucose. Control non-glycated BSA (2.0 g in 10 ml phosphate-buffered saline) was prepared with the same procedure without D-glucose inclusion. AGE-BSA content was estimated by fluorescence spectroscopy (excitation at 390 nm and emission at 460 nm) at a protein concentration of 1 mg/ml, which indicated a 10-fold increase in fluorescence in AGE-BSA compared with control BSA. The prepared AGE-BSA and unmodified BSA were sterilized by ultrafiltration and stored at −20 °C until use. Endotoxin levels of each preparation were measured by Limulus amoebocyte lysate assay (E-Toxate, Sigma, St Louis, MO, USA) and were found to be <0.8 EU/ml.
Cell Culture and Treatments
Rat VSMCs were isolated using explant culture method as described previously.18 Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Invitrogen), penicillin (100 U/ml), and streptomycin (100 μg/ml) and incubated at 37 °C in a humidified atmosphere with 5% CO2. Cells at 70% confluence were starved for 24 h in DMEM containing 0.1% FBS and were treated with anti-RAGE antibody (5 μg/ml), the KCa3.1 channel blocker TRAM-34 (100 nM), the ERK1/2 inhibitors PD98059 (25 μM) and U0126 (10 μM), the P38-MAPK inhibitors SB203580 (10 μM) and SB202190 (10 μM), and the PI3K inhibitors LY294002 (10 μM) and wortamannin (100 nM) for 30 min. Later, AGE-BSA (200 μg/ml) was added into the medium containing 0.1% FBS for continuous culture.
Electrophysiology
Membrane current was recorded in cultured VSMCs with the whole-cell patch-clamp technique as described previously.21 The detached VSMCs were placed into a 0.2-ml cell chamber mounted on inverted microscope, allowed to settle to the bottom (∼20 min), and then superfused with Tyrode solution (pH 7.4) containing (mM): 136 NaCl, 5.4 KCl, 1.0 MgCl2, 1.8 CaCl2, 10 glucose, and 10 HEPES. Borosilicate glass electrodes (1.2 mm outer diameter) were pulled with a Brown-Flaming puller (Model P-97; Sutter Instrument, Novato, CA, USA) and had a resistance of 2–3 MΩ when filled with pipette solution containing (mM): 120 K-aspartate, 20 KCl, 1.0 MgCl2, 5.0 EGTA, 5 Na-phosphocreatine, 5 Mg-ATP, 0.1 GTP, 10 HEPES, 500 nM free Ca2+ with pH adjusted to 7.2 with KOH. The tip potentials were zeroed before the pipette contacted the cell. After a gigaohm seal was obtained by negative suction, the cell membrane was ruptured by a gentle suction to establish whole-cell configuration. Membrane currents were recorded with an EPC-9 amplifier and Pulse software (Heka Elektronik, Lambrecht, Germany). Command pulses were generated by a 12-bit digital-to-analog converter controlled by Pulse software. All current recording experiments were conducted at room temperature (22–23 °C).
Western Blot Analysis
VSMCs incubated with AGE-BSA in the presence or absence of indicated inhibitors for 24 h (for KCa3.1 immunoblotting) or 30 min (for ERK1/2, phospho-specific ERK1/2, P38, phospho-specific P38, Akt, and phospho-specific Akt immunoblotting) were lysed with ice-cold modified RIPA buffer (60 mM Tris-HCl, 0.25% SDS, 1 mM sodium fluoride, 1 mM sodium orthovanadate, and 2 μg/ml aprotinin and leupeptin). The lysates were then centrifuged at 12 000 g for 15 min at 4 °C. After transferring the supernatant to a fresh ice-cold tube, the protein concentration was determined with BCA protein assay. Equal concentrations of proteins were mixed with SDS sample buffer and denatured at 100 °C for 5 min. The samples were separated on an SDS-10% polyacrylamide gel, then transferred to a PVDF membrane at 300 mA for 2 h in a transfer buffer containing 20 mM Tris, 150 mM glycine, and 20% methanol. The membranes were blocked with 1% BSA in TBST (0.1% Tween-20) for 1 h. After blocking, the blots were incubated in primary antibody for KCa3.1 (1:500), anti-ERK1/2 (1:500), phospho-specific anti-ERK1/2 (p-ERK1/2, 1:500), P38 (1:200), phospho-specific anti-P38 (p-P38, 1:200), anti-Akt (1:500), and phospho-specific anti-Akt (1:500) at 4 °C overnight and then incubated with HRP-conjugated secondary antibodies (1:5000) for 1 h at room temperature. The bound antibodies were detected with an enhanced chemiluminescence detection system (ECL, GE Biotech, USA) and quantified by densitometry using a Chemi-Genius Bio Imaging System (Syngene, Cambridge, UK). To ensure equal sample loading, the ratio of band intensity to GAPDH was obtained to quantify the relative protein expression level.
RNA Interference
Stealth RNAi molecules targeted to KCa3.1 channels were purchased from Invitrogen Life Technology (Invitrogen, USA). Specific small interfering RNA (siRNA) sequences of KCa3.1 were as follows:22 sense: 5′-GCCACUGGUUCGUGGCCAAACUAUA-3′, antisense: 5′-UAUAGUUUGGCCACGAACCAGUGGC-3′. In addition, Silencer GAPDH siRNA (Ambion, Austin, TX, USA) was used as the positive control. Stealth RNAi molecules at 100 nM were transfected into the rat VSMCs at 50% confluence for 24 h using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Stealth RNAi of medium GC content (Invitrogen), which had no known target in mammalian genomes, was used as the control. Transfected cells were used for cell migration and proliferation assay or protein extraction after 24 h of transfection. Transfection efficiency was monitored using fluorescent RNA duplex (Invitrogen) according to the manufacturer’s instructions.
Cell Migration Assay
The migration of VSMCs was determined by modified Boyden chamber technique.23 Briefly, a 24-well Transwell apparatus with each well containing a 6.5-mm polycarbonate membrane with 8 μm pores was used. Serum-starved or transfected VSMCs were trypsin-harvested. Cell suspension in basal medium (250 μl, 1 × 105 cells/well or 5 × 104 cells/well in RNAi) was seeded in upper chamber, and 750 μl of basal medium with various reagents was added to the lower chamber. After incubation for 24 h, cells were removed from the top side of the membrane, and the migrated cells from the reverse side were stained with hematoxylin and then quantified. Each treatment was repeated in six independent transwells.
Cell Proliferation Assays
The cell proliferation was determined by cell count and BrdU incorporation assay as previously described.16 In cell-counting experiment, rat VSMCs were plated in 24-well plates at a density of 1 × 105 cells per well (or 1 × 104 cells per well in RNAi) in 1 ml DMEM containing 10% FBS. After starving for 24 h or 6 h (in RNAi) in DMEM containing 0.1% FBS, the cells were pretreated by KCa3.1 channel blocker or anti-RAGE antibody for 30 min, or KCa3.1 channel siRNA for 24 h before AGE-BSA was added. Following 24 h culture, cells were detached with the solution containing trypsin (0.25%) and EDTA (0.02%) and counted using a standard hemocytometer in a blinded manner. Cell viability was established by trypan blue exclusion. In BrdU-labeling detection, cells grown on coverslips were treated as indicated in the figures and labeled with 10 μM BrdU for 24 h at 37 °C. The coverslips were then washed with phosphate-buffered saline, fixed in 4% paraformaldehyde for 20 min at room temperature, and DNA was denatured with 2 N HCl for 30 min in an incubator at 37 °C. After incubation in blocking buffer (1% normal BSA and 0.1% Tween-20) for 1 h, the cells were immunostained with mouse anti-BrdU (1:1000) primary antibody overnight in a moist chamber at 4 °C followed by appropriated Rhodamine-labeled goat anti-mouse secondary antibody. Nuclear DNA was counterstained with 4,6-diamidino-2-phenylindole. Images were then captured with a microscope (Olympus IX71, Tokyo, Japan). The total number of nuclei (blue color) and the number of BrdU-stained nuclei (pink color) were counted manually. Proliferation was quantitated as percentage of BrdU-labeled cells in the total number of cells counted.
Statistical Analysis
All quantitative measures are presented as mean±s.e.m. Paired and/or unpaired Student’s t-tests were used as appropriate to evaluate the statistical significance of differences between two group means, and analysis of variance (ANOVA) was performed for multiple groups. P<0.05 was considered statistically significant.
RESULTS
AGEs and K Ca 3.1 Current in Cultured Rat VSMCs
Figure 1a shows the membrane currents recorded with 300-ms voltage steps between −100 mV and +60 mV from a holding potential of −70 mV (inset) in cells treated with BSA (200 μg/ml), AGE-BSA (200 μg/ml), and AGE-BSA plus anti-RAGE antibody (5 μg/ml) before and after the application of the KCa3.1 channel blocker TRAM-34. The current exhibited a weak inward rectification at positive potentials and was sensitive to the full inhibition by 1 μM TRAM-34, indicating that the KCa3.1 channels are predominantly expressed in cultured rat VSMCs. KCa3.1 current was remarkably enhanced in cells treated with AGE-BSA for 24 h. It is interesting to note that the increased KCa3.1 current was antagonized in cells treated with both AGE-BSA and anti-RAGE antibody. Figure 1b illustrates the mean values of current–voltage (I–V) relationships of TRAM-34-sensitive current obtained by digital subtraction of the current before TRAM-34 by the current after TRAM-34 application. The I–V curves of TRMA-34-sensitive showed a weak inward rectification. The current density at −100, −90 mV, and −50 to +60 mV was greater in cells treated with 200 μg/ml AGE-BSA (n=6, P<0.05 or P<0.01 vs control). The enhanced current by AGE-BSA was almost fully antagonized by anti-RAGE antibody (n=6, P<0.05 or P<0.01 vs AGE-BSA alone at −50 to +60 mV). These results indicate that the increase of KCa3.1 current by AGE-BSA is mediated by RAGE in cultured rat VSMCs.
Effect of AGE-BSA on KCa3.1 currents in cultured rat VSMCs. (a) Membrane currents recorded with the protocol as shown in the inset in cells treated with BSA (200 μg/ml), AGE-BSA (200 μg/ml), and AGE-BSA plus anti-RAGE antibody (5 μg/ml) for 24 h before and after application of TRAM-34 (1 μM). (b) I–V relationships of the mean values of TRAM-34-sensitive current obtained by digital subtraction of the current before TRAM-34 by the current after TRAM-34 application (n=6 for each groups).
AGEs and K Ca 3.1 Channel Expression in VSMCs
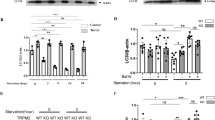
To determine whether the increase of KCa3.1 current by AGE-BSA is related to an enhanced expression of the channel, the protein level of KCa3.1 channels was examined in cultured VSMCs. Figure 2 shows the images of western blot and mean values of relative protein levels of KCa3.1 channels in cells treated with vehicle (control), BSA, AGE-BSA, AGE-BSA with anti-RAGE antibody, or different protein kinase-related inhibitors. AGE-BSA (200 μg/ml), but not BSA (200 μg/ml), increased KCa3.1 channel protein expression, the effect was countered by 5 μg/ml anti-RAGE antibody (Figure 2a) or the PI3K/Akt inhibitors LY294002 (10 μM) and wortmannin (100 nM, Figure 2b), the ERK1/2 inhibitors PD98059 (25 μM) and U0126 (10 μM, Figure 2c), and the P38-MAPK inhibitors SB203580 (10 μM) and SB203580 (10 μM, Figure 2d, n=5, P<0.01 vs control or BSA, P<0.01 vs AGE-BSA alone). KCa3.1 channel protein was not influenced by application of protein kinase-related inhibitors alone. These results demonstrate that the increase of KCa3.1 current by AGEs is related to the upregulation of the channel protein in cultured rat VSMCs, and ERK1/2, P38-MAPK, as well as PI3K/Akt signals participate in the regulation of KCa3.1 expression.
Effect of AGE-BSA on protein expression of KCa3.1 channels in rat VSMCs. (a) Western blots and mean values of KCa3.1 channel protein in cells treated without (control) or with 200 μg/ml BSA, 200 μg/ml AGE-BSA, and AGE-BSA plus 5 μg/ml anti-RAGE antibody for 24 h (n=5, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone). (b) Western blots and mean values of KCa3.1 channel protein in cells treated without (control) or with 200 μg/ml BSA, 200 μg/ml AGE-BSA, AGE-BSA plus 10 μM LY294002 or 100 nM wortmannin, and LY294002 or wortmannin alone for 24 h (n=5, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone). (c) Western blots and mean values of KCa3.1 channel protein in cells treated without (control) or with 200 μg/ml BSA, 200 μg/ml AGE-BSA, AGE-BSA plus 25 μM PD98059 or 10 μM U0126, and PD98059 or U0126 alone for 24 h (n=5, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone). (d) Western blots and mean values of KCa3.1 channel protein in cells treated without (control) or with 200 μg/ml BSA, 200 μg/ml AGE-BSA, AGE-BSA plus 10 μM SB203580 or 10 μM SB202190, and SB203580 or SB202190 alone for 24 h (n=5, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone).
AGEs and Intracellular Signal Activity
The above results suggest that ERK1/2, P38-MAPK, and PI3K are involved in the upregulation of KCa3.1 channel expression by AGEs. To determine whether the phosphorylation of these signals are affected by AGEs, western blot analysis was used to determine total and phosphorylated levels of ERK1/2, P38, and Akt kinases in VSMCs treated without (control) or with BSA (200 μg/ml), AGE-BSA (200 μg/ml), and AGE-BSA plus anti-RAGE antibody (5 μg/ml). Figure 3 shows the western blots and the mean values of total and phosphorylated ERK1/2, P38, and Akt. It is interesting to note that AGEs significantly increased phosphorylated ERK1/2, P38, and Akt (n=4-5, P<0.05 vs control or BSA), and the effect was fully antagonized by anti-RAGE antibody (P<0.05 vs AGE-BSA alone). These results indicate that the upregulation of KCa3.1 channels induced by AGEs are related to the RAGE-mediated activation of ERK1/2, P38, or Akt in cultured rat VSMCs.
AGE-BSA and phosphorylation of ERK1/2, Akt, and P38 kinases in rat VSMCs. (a) Western blots and mean values of p-ERK1/2 in cells treated without (control) or with BSA (200 μg/ml), AGE-BSA (200 μg/ml), or AGE-BSA and anti-RAGE antibody (5 μg/ml) for 30 min (n=5, *P<0.05 vs control or BSA; #P<0.05 vs AGE-BSA alone). (b) Western blots and mean values of p-Akt in cells treated with the interventions as described in a (n=4, *P<0.05 vs control or BSA; #P<0.05 vs AGE-BSA alone). (c) Western blots and mean values of p-P38-MAPK in cells treated with the interventions as described in a. (n=4, *P<0.05 vs control or BSA; #P<0.05 vs AGE-BSA alone).
Role of AGEs in Regulating Migration of Rat VSMCs
To investigate whether the upregulation of KCa3.1 channels by AGE-BSA would influence cell function, we tested the effect on migration in cultured VSMCs. Figure 4 illustrates the cell migration as determined by Boyden chamber assay. AGE-BSA (200 μg/ml), but not BSA (200 μg/ml), increased cell migration by 64% (n=6, P<0.01 vs control or BSA), and the effect was countered by 100 nM TRAM-34 or 5 μg/ml anti-RAGE antibody (Figure 4a, n=6, P<0.01 vs AGE-BSA alone). These results indicate that AGEs-induced cell migration is likely mediated by KCa3.1 channels.
Effects of AGE-BSA and KCa3.1 channels on cell migration in rat VSMCs. (a) Mean values of migrated cell number determined with Boyden chamber technique in cells treated with 200 μg/ml BSA, 200 μg/ml AGE-BSA, AGE-BSA plus 100 nM TRAM-34, or 5 μg/ml anti-RAGE antibody for 24 h (n=6, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone). (b) Western blots of KCa3.1 channels in cells transfected with 100 nM control siRNA or KCa3.1 siRNA for 24 h. (c) Mean values of migrated cell number in cells transfected with control siRNA or KCa3.1 siRNA (100 nM) and treated with 200 μg/ml AGE-BSA or 200 μg/ml BSA (n=6, **P<0.01 vs control siRNA with BSA; ##P<0.01 vs control siRNA with AGE-BSA).
To rule out the possible non-specific effect of the KCa3.1 channel blocker TRAM-34 on cell migration, the test was performed in cells treated with the specific siRNA targeting KCa3.1 channels. The protein level of KCa3.1 channel was reduced in cells transfected with KCa3.1 siRNA (100 nM) but not with control siRNA (Figure 4b). AGE-BSA significantly increased the migration in cells transfected with control siRNA but not with KCa3.1 siRNA (Figure 4c, n=6, P<0.01 vs BSA plus control siRNA; P<0.01 vs AGE-BSA plus control siRNA). These results further suggest that AGEs-induced cell migration was mediated by KCa3.1 channels in rat VSMCs.
Role of AGEs in Regulating Proliferation of Rat VSMCs
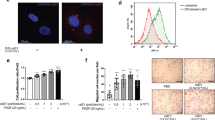
The effect of AGEs on proliferation was determined by cell counting and BrdU incorporation assay in cultured VSMCs. Figure 5a displays the cell number counted in cells incubated with BSA (200 μg/ml), AGE-BSA (200 μg/ml), AGE-BSA plus TRAM-34 (100 nM), and anti-RAGE antibody (5 μg/ml). The cell number was increased by 41.2% with AGE-BSA (n=6, P<0.01 vs control or BSA), and the effect was antagonized by TRAM-34 or anti-RAGE antibody (n=6, P<0.01 vs AGE-BSA alone). Figure 5b illustrates the images of VSMCs stained with anti-BrdU antibody, showing the newly synthesized DNA. The cells were incubated with AGE-BSA (200 μg/ml) for 24 h in the absence or presence of TRAM-34 or anti-RAGE antibody. AGE-BSA, but not BSA, significantly increased the number of cells with BrdU-stained nuclei, and the effect was countered by TRAM-34 or anti-RAGE antibody (Figure 5c, n=6, P<0.01 vs AGE-BSA alone). These results suggest that AGEs-RAGE interaction promotes cell proliferation through KCa3.1 channels in cultured rat VSMCs.
AGE-BSA increases cell proliferation by activating KCa3.1 channels in rat VSMCs. (a) Mean values of cell counting in cells treated without (control) or with BSA (200 μg/ml), AGE-BSA (200 μg/ml), and AGE-BSA plus 100 nM TRAM-34 or 5 μg/ml anti-RAGE antibody 24 h (n=6, **P<0.01 vs control or BSA, ##P<0.01 vs AGE-BSA alone). (b) Immunofluorescent BrdU-staining images ( × 100) of the nuclei in VSMCs treated with the interventions as described in a. Nuclei counterstained in blue (4,6-diamidino-2-phenylindole) and BrdU-positive nuclei shown in pink. No visible pink color (the color of rhodamine-labeled second antibody) was observed in negative control without incubation of the primary antibody. (c) Mean percent values of BrdU-incorporated nuclei in VSMCs treated with the interventions as described in a. (n=6, **P<0.01 vs control or BSA; ##P<0.01 vs AGE-BSA alone). (d) Mean values of cell number counted in cells transfected with control siRNA or KCa3.1 siRNA (100 nM) and treated with BSA or AGE-BSA (200 μg/ml) for 24 h (n=6, **P<0.01 vs control siRNA with BSA; ##P<0.01 vs control siRNA with AGE-BSA). (e) BrdU-incorporation staining ( × 100) in cells treated with the interventions as described in d. (f) Mean percentage of values of proliferation cells with pink color in cells treated with the interventions as described in d (n=6, **P<0.01 vs control siRNA with BSA; ##P<0.01 vs control siRNA with AGE-BSA).
To further study the involvement of KCa3.1 channels in AGEs-mediated stimulation of proliferation, cell proliferation was determined in cells transfected with control siRNA or KCa3.1 siRNA. Figure 5d illustrates the effects of the specific KCa3.1 siRNA (100 nM) on proliferation. The cell number was increased by 111.5% in AGE-BSA-treated cells with control siRNA transfection (n=6, P<0.01 vs BSA), and the effect was antagonized by the downregulation of KCa3.1 channels (n=6, P<0.01 vs control siRNA with AGEs). Figure 5e shows the BrdU-stained cells treated with BSA (200 μg/ml) or AGE-BSA (200 μg/ml). In cells transfected with control siRNA, AGE-BSA increased cell proliferation by 624%, and the effect was significantly reduced in cells transfected with KCa3.1 siRNA (Figure 5f, n=6, P<0.01 vs control siRNA). These results demonstrate that KCa3.1 channels are involved in enhancement of cell proliferation by AGEs in rat VSMCs.
DISCUSSION
In the present study, we demonstrate for the first time that AGE-BSA upregulates KCa3.1 channels in cultured VSMCs and increases cell migration and proliferation, which is involved in the activation of ERK1/2, P38-MAPK, and PI3K signaling pathways. This study provides the novel information that AGEs are likely involved, at least in part, in the excessive neointimal VSMC proliferation and the increased KCa3.1 channel expression in the aorta of diabetic rats.18
AGEs are the products generated from nonenzymatic glycation and oxidation of proteins and lipids. Persistent hyperglycemia and oxidative stress accelerate the formation of AGEs in diabetic subjects, which has an important role in the development of accelerated atherosclerosis. AGEs can induce VSMC proliferation and migration although the mechanisms remain not fully understood. Upregulation of KCa3.1 channels has been found to promote excessive VSMC proliferation and migration induced by either mitogens (platelet-derived growth factor and EGF) or balloon catheter injury.13, 14, 15 However, it is unknown whether AGEs is involved in the KCa3.1 channel regulation. In the present study, we demonstrated that AGE-BSA increased KCa3.1 current density and enhanced KCa3.1 channel protein expression in cultured rat VSMCs. In addition, we found that AGE-BSA increased cell migration and proliferation. These effects were antagonized by anti-RAGE antibody, the KCa3.1 channel blocker TRAM-34, or KCa3.1 siRNA. These results provide a direct link between AGEs and KCa3.1 channels in the regulation of VSMC migration and proliferation.
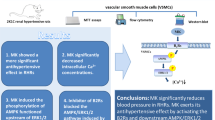
It is generally recognized that cellular effects of AGEs were mediated by the specific cellular receptor RAGE (Figure 6). Previous studies showed that the interaction of AGEs with RAGE in rat VSMCs leads to an increased phosphorylation of ERK1/2, P38-MAPK,9, 4 Src kinases10 and promotes cell proliferation and migration. The phosphorylation of PI3K is also essential for RAGE ligand-induced VSMC migration and proliferation.24 In the present study, we found that specific signal inhibitors, PD98059 and U0126 for ERK1/2, SB203580 and SB202190 for P38-MAPK, and LY294002 and wortmannin for PI3K/Akt, markedly decreased AGEs-induced upregulation of KCa3.1 channel expression in rat VSMCs, indicating that activation of these cellular signaling pathways mediate the AGEs-induced increase of KCa3.1 channels. This is supported by previous reports, in which ERK induces an increase of proliferative gene expression, eg, the AP-1 family member c-fos,25, 26, 27 and the increased production of c-fos leads to induction of KCa3.1 expression through AP-1 promoter elements.28
Schematic graph showing the effects of AGEs and insulin on intracellular pathways. AGEs bind to their receptor RAGE and activate P38, ERK1/2, and PI3K/Akt and then promote gene expression of KCa3.1 channels, which has a crucial role in regulating migration and proliferation in VSMCs. Insulin regulates cell proliferation and migration via a similar mechanism.
In addition to the upregulation of KCa3.1 current and KCa3.1 expression by AGEs, the recent studies reported that KCa3.1 channels were activated by PI(3)P, which is produced by PI3K from phosphatidylinositol.29, 30, 31 Decreasing intracellular level of PI(3)P can reduce KCa3.1 channel activity.32 Besides, recombinant human KCa3.1 channels induce HEK293 cell proliferation by a direct interaction with ERK1/2 pathways33 and downregulating ERK reduces KCa3.1 channel expression.32 These reports support the notion that KCa3.1 channels are the downstream target of ERK1/2, P38-MAPK and PI3K in regulating cell proliferation and migration of rat VSMCs (Figure 6).
It is believed that atherosclerosis involves a switch to a proliferative phenotype in VSMCs. KCa3.1 channels are the predominant Ca2+-activated K+ channels in proliferative smooth muscle cells but not in mature contractile cells.11, 12, 13, 14 The expression of KCa3.1 channels is increased in VSMCs in coronary arteries from patients and in aortas of ApoE−/− mice with atherogenesis, and KCa3.1 channel blocker markedly slows down the development of atherosclerosis in ApoE−/− mice.16 However, reports related to the regulation of KCa3.1 channels are limited in diabetes. Our results suggest that AGEs, as insulin,18 have a crucial role in upregulating KCa3.1 channel activity and expression in VSMCs (Figure 6). More effort is required in the future to investigate whether blockade of AGEs signal with RAGE antibody or specific KCa3.1 channel blocker would be beneficial in managing diabetic vascular complications in vivo.
In summary, the results of the present study provide the novel information that AGEs increase the expression of KCa3.1 channels in a RAGE-dependent manner by activating ERK1/2, P38-MAPK and PI3K signal pathways and therefore promote VSMC migration and proliferation, which may have a role in the development of diabetic vascular complications.
References
Singh R, Barden A, Mori T et al. Advanced glycation end-products: a review. Diabetologia 2001;44:129–146.
Hudson BI, Schmidt AM . RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res 2004;21:1079–1086.
Brownlee M . Advanced protein glycosylation in diabetes and aging. Annu Rev Med 1995;46:223–234.
Burke AP, Kolodgie FD, Zieske A et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 2004;24:266–1271.
Bucciarelli LG, Wendt T, Qu W et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E null mice. Circulation 2002;106:2827–2835.
Wendt T, Harja E, Bucciarelli L et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis 2006;185:70–77.
Soro-Paavonen A, Watson AM, Li J et al. Receptor for advanced glycation endproducts (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008;57:2461–2469.
Sakaguchi T, Yan SF, Yan SD et al. Central role of RAGE-dependent neointimal expansion. J Clin Invest 2003;111:959–972.
Yoon YW, Kang TS, Lee BK et al. Pathobiological role of advanced glycation endproducts via mitogen-activated protein kinase dependent pathway in the diabetic vasculopathy. Exp Mol Med 2008;40:398–406.
Reddy MA, Li SL, Sahar S et al. Key role of Src kinase in S100b-induced activation of the receptor for advanced glycation endproducts in vascular smooth muscle cells. J Biol Chem 2006;281:13685–13693.
Neylon CB, Avdonin PV, Larsen MA et al. Rat aortic smooth muscle cells expressing charybdotoxin-sensitive potassium channels exhibit enhanced proliferative responses. Clin Exp Pharmacol Physiol 1994;21:117–120.
Neylon CB, Lang RJ, Fu Y et al. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res 1999;85:e33–e43.
Si H, Grgic I, Heyken WT et al. Mitogenic modulation of Ca2+-activated K+ channels in proliferating A7r5 vascular smooth muscle cells. Br J Pharmacol 2006;148:909–917.
Tharp DL, Wamhoff BR, Turk JR et al. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol 2006;291:H2493–H2503.
Kohler R, Wulff H, Eichler I et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 2003;108:1119–1125.
Toyama K, Wulff H, Chandy G et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest 2008;118:3025–3037.
Wang Y, Zhang HT, Su XL et al. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovas Res 2010;7:75–84.
Su XL, Wang Y, Zhang W et al. Insulin-mediated upregulation of KCa3.1 channels promotes cell migration and proliferation in rat vascular smooth muscle. J Mol Cell Cardiol 2011;51:51–57.
Nyhlin N, Ando Y, Nagai R et al. Advanced glycation end product in familial amyloidotic polyneuropathy. J Intern Med 2000;247:485–492.
Horiuchi S, Araki N, Morino Y . Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J Biol Chem 1991;266:7329–7332.
Tao R, Lau CP, Tse HF et al. Regulation of cell proliferation by intermediate-conductance Ca2+-activated potassium and volume-sensitive chloride channels in mouse mesenchymal stem cells. Am J Physiol Cell Physiol 2008;295:C1409–C1416.
Deng XL, Lau CP, Lai K et al. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif 2007;40:656–670.
Chung CH, Lin KT, Chang CH et al. The integrin α2β1 agonist, aggretin, promotes proliferation and migration of VSMC through NF-kB translocation and PDGF production. Br J Pharmacol 2009;156:846–856.
Touré F, Fritz G, Li Q et al. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res 2012;110:1279–1293.
Stevenson AS, Cartin L, Wellman TL et al. Membrane depolarization mediates phosphorylation and nuclear translocation of CREB in vascular smooth muscle cells. Exp Cell Res 2001;263:118–130.
Taubman MB, Berk BC, Izumo S et al. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem 1989;64:526–530.
Pulver RA, Rose-Curtis P, Roe MW et al. Store-operated Ca2+ entry activates the CREB transcription factor in vascular smooth muscle. Circ Res 2004;94:1351–1358.
Ghanshani S, Wulff H, Miller MJ et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem 2000;275:37137–37149.
Lee BH, Bae JS, Park RW et al. βig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through αvβ5 integrin. Exp Mol Med 2006;38:153–161.
Srivastava S, Li Z, Ko K et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell 2006;24:665–675.
Di L, Srivastava S, Zhdanova O et al. Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. J Biol Chem 2010;285:38765–38767.
Srivastava S, Di L, Zhdanova O et al. The class II phosphatidylinositol 3 kinase C2β is required for the activation of the K+ channel KCa3.1 and CD4 T-cells. Molecular. Biol Cell 2009;20:3783–3791.
Park S, Kim JA, Joo KY et al. Globotriaosylceramide leads to KCa3.1 channel dysfunction: a new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res 2011;89:290–299.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant numbers 81070129 and 81100231) and the Doctor Science Foundation of China (Grant number 200806980032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Advanced glycation endproducts (AGEs) increase the expression of intermediateconductance Ca2+-activated K+ channels in an AGE receptor-dependent manner by activating ERK1/2, P38-MAPK and PI3K signal pathways. AGEs therefore promote vascular smooth muscle cell migration and proliferation, which may play a role in the development of diabetic vascular complications.
Rights and permissions
About this article
Cite this article
Zhao, LM., Su, XL., Wang, Y. et al. KCa3.1 channels mediate the increase of cell migration and proliferation by advanced glycation endproducts in cultured rat vascular smooth muscle cells. Lab Invest 93, 159–167 (2013). https://doi.org/10.1038/labinvest.2012.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2012.163
Keywords
This article is cited by
-
Advanced Glycation End Products: key player of the pathogenesis of atherosclerosis
Glycoconjugate Journal (2022)
-
Mechanisms underlying suppression of noradrenaline-induced contraction by prolonged treatment with advanced glycation end-products in organ-cultured rat carotid artery
Pflügers Archiv - European Journal of Physiology (2020)
-
Amplification of the COX/TXS/TP receptor pathway enhances uridine diphosphate-induced contraction by advanced glycation end products in rat carotid arteries
Pflügers Archiv - European Journal of Physiology (2019)
-
Metformin regulates atrial SK2 and SK3 expression through inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats
BMC Cardiovascular Disorders (2018)
-
Diabetes modifies the role of prostanoids and potassium channels which regulate the hypereactivity of the rabbit renal artery to BNP
Naunyn-Schmiedeberg's Archives of Pharmacology (2018)